| Chapter 6: Reactions of Alkenes : Addition Reactions |
| Chapter 6: Reactions of Alkenes : Addition Reactions |
Addition of non-symmetrical reagent (i.e. A-B where A is not equal to B) to a non-symmetrical
alkene (i.e. where the groups at each end of the double bond are different), gives two isomeric products that are constitutional isomers.
Since the structures differ only in the location of the substituents, they are positional isomers which are also referred to as regioisomers.
For example, the reaction of HCl with propene gives the regioisomers 2-chloropropane and 1-chloropropane.
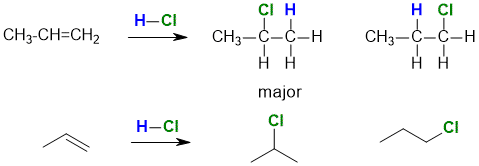
We will talk about this particular reaction in more
detail later.
Stereoisomers are molecules that have the same groups connected in the same order (i.e. same connectivity), except for the arrangement of those bonds in 2D or 3D space.
When an alkene undergoes addition, two new s
bonds are formed. If we think of an alkene as having two faces, then the two
new s bonds can either both form on the same face,
which we call syn addition, or they can be formed on different faces
which we call anti addition. Do you remember these terms from Chapter
3 (conformational analysis) ? Therefore the new bonds that are being formed are being formed in different directions in 3D space.
A simplified illustration is provided below along with the corresponding interactive JSMOL models.
Remember that an alkene unit is planar. The alkene has been drawn in a wedge hash type style which means that the substituents on the C=C are into and out of the page with the C=C unit horizontal. This means that the two faces of the alkene are above and below, or a top face and bottom face in this convenient arrangement.
Syn addition results when the two new bonds are both formed to the same face of the alkene, in this case they are shown to have formed on the top face.
Anti addition results when the two new bonds have formed to opposite faces, one to the top face, the other to the lower face.
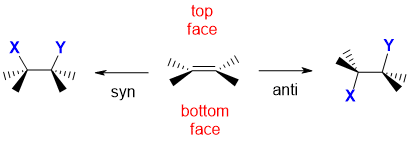
new bonds to X and Y have formed to the same face |
new bonds to X and Y have formed to opposite faces |
Note : This is a simplification....
In the case of a very simple alkene like ethene, the free rotation
of the C-C bond in the addition product means that the two product structures are conformational
isomers (a type of stereoisomer) and rapidly interconvert.
With other acyclic alkenes, the free rotation
of the C-C bond in the addition product means one needs to look carefully at the stereoisomer products to deduce to the
nature of the addition process.
However with cyclic alkenes (see below), where the ring structure prevents the free rotation, the two products
are stereoisomers and are more obvious because the added groups are either on the same face (from syn addition) or opposite faces (from anti addition):
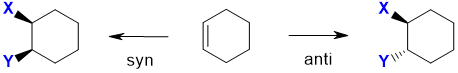
| © Dr. Ian Hunt, Department of Chemistry |