| Chapter 7 : Stereochemistry |
| Chapter 7 : Stereochemistry |
If a pair of stereoisomers
are non-superimposable mirror images of each other, then they are enantiomers.
Look at the pair of JSMOL images of molecules shown below and make sure you can see
this. This particular example was chosen so that each "group" is a simple coloured
sphere.
If you don't know what is meant by non-superimposable mirror images.... the first step is to stop thinking about molecules and just think about any objects as this is a geometric issue and not limited to chemistry and is really something that we deal with everyday of our lives. There are two parts to what we need to consider, mirror image and superimposability
For example, if you look at your hands, you will see that while they are very similar (i.e. made up of the same peices, on each hand there are 4 fingers and a thumb, a wrist, fingertips, a palm and the back of the hand)... but they are not the same. To a first approximation, they are mirror images of each other. However, they can not be superimposed, you can always tell a left from a right (I hope !). Therefore, since your left and right hands are non-superimposable mirror images, then they are a pair of enantiomers.
The two enantiomers of 2-chlorobutane are shown below.
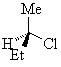
(R)-2-chlorobutane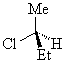
(S)-2-chlorobutane
Questions
A molecule that can exist as a pair of enantiomers has the property of chirality (derived from the Greek meaning handedness), or is described as chiral. A molecule that lacks this property is achiral.
You should be able to draw the two mirror images of an achiral molecule and then see how if you rotate one of the images the two molecules are actually the same (i.e. that they are superimposable). For example, compare 2-chloropropane and 2-chlorobutane systems :
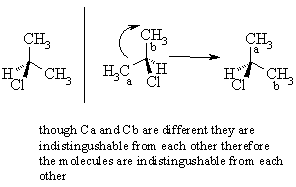
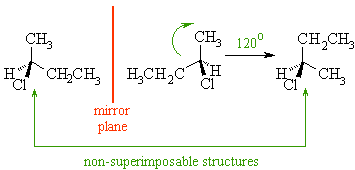
Because the 2 alkyl groups are indistinguishable, the original molecule and its' mirror image are superimposable. Because the 2 alkyl groups are distinguishable, the original molecule and its' mirror image are non-superimposable.
Important properties of enantiomers that you should know: Other things to know:
- Same physical properties except that they rotate plane polarised light in opposite directions (see later).
- Same chemical properties except when another chiral molecule is involved.
A mixture that contains equal quantities of enantiomers is called a racemate or a racemic mixture.
| © Dr. Ian Hunt, Department of Chemistry |