| Chapter 9: Alkynes |
| Chapter 9: Alkynes |
Halogenation of Alkynes
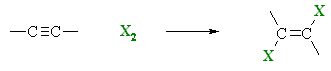
Summary
| This JSMOL image shows the structure of
the bromonium ion formed during the reaction of ethyne with Br2.
Note how the Br is attached to both the C atoms. QUESTION What is the charge on the bromine ?ANSWER When the Br- nucleophile attacks, it will attack from the least hindered face, the side opposite to the Br in this intermediate. Make sure you look at the spacefilling model to show this. |
| MECHANISM FOR REACTION OF ALKYNES
WITH HALOGENS |
|
| Step 1: The π electrons act as a nucleophile, attacking the bromine, displacing a bromide ion but forming a cyclic bromonium ion as an intermediate. |
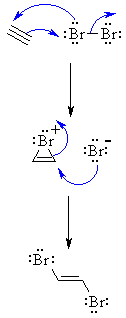 |
| Step 2: Attack of the nucleophilic bromide from the side away from the bromonium center in an SN2 like fashion opens the cyclic bromonium ion to give overall anti addition. |
|
| © Dr. Ian Hunt, Department of Chemistry |