| Chapter 9 : Alkynes |
| Chapter 9 : Alkynes |
| Qu 1: | The π bond in an alkene is given by : C=C - C-C |
| 611 kJ/mol
(146 kcal/mol) - 368 kJ/mol (88 kcal/mol) = 243
kJ/mol (58 kcal/mol) |
|
| The second π bond in an alkyne is given by : C≡C - C=C | |
| 820 kJ/mol
(196 kcal/mol) - 611 kJ/mol (146 kcal/mol) = 209 kJ/mol (50
kcal/mol)
This indicates that it should be recognised that the extra π bond in an alkyne is a weaker bond than the p bond in an alkene. |
|
| Qu 2: | Consider the following points about the reaction of 2-butyne with HBr |
| (a) Since the reaction gives predominantly the 2-bromo-2-butene, the product must be less reactive than the alkyne starting material otherwise a mixture the mono-bromide and the dibromide would be formed. | |
(b) 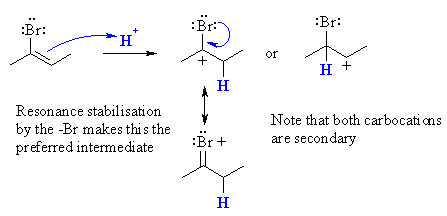 Protonation of 2-bromo-2-butene gives a secondary carbocation regardless of which of the two C get protonated. However, since the only product is 2,2-dibromobutane, the intermediate must be that from the carbocation with the +ve charge formed adjacent to the bromine. This can be justified by considering the resonance stabilising influence the bromine can provide. |
|
| Qu 3: | The final product is 2-butyne. |
| The steps are: (i) propene to 1,2-dibromopropane (addition) then (ii) elimination to propyne, a terminal alkyne, and (iii) removal of the terminal H gives the carbanion which undergoes an SN2 with the methyl iodide forming a new CC bond. | |
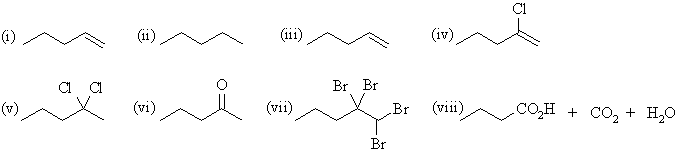
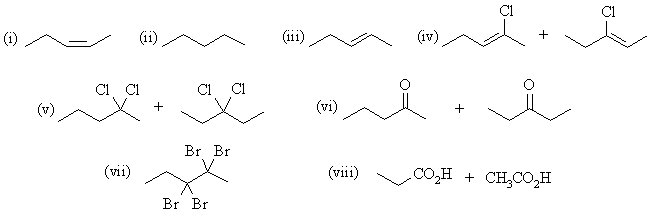
| Qu 4: | The major products are: |
(a) 1-pentyne
|
|
(b) 2-pentyne
|
| © Dr. Ian Hunt, Department of Chemistry |