| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
| Chapter 10: Conjugation in Alkadienes and Allylic Systems |
π Molecular Orbitals of 1,3-Butadiene
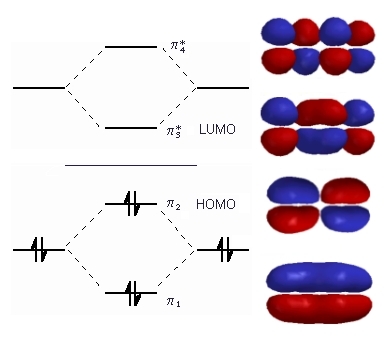
| The diagram to the right shows the relative energies
of the π molecular orbitals of 1,3-butadiene
(derived from ethene) and the electron configuration.
The horizontal center line denotes the energy of a C atomic p orbital. Orbitals below that line are bonding those above are anti-bonding. We now have 4 electrons to arrange, 1 from each of the original atomic p orbitals. These are all paired in the two stabilised pi bonding orbitals, π1 and π2. The highest occupied molecular orbital or HOMO is π2 in 1,3-butadiene (or any simple conjugated diene). In contrast, the anti-bonding π* orbitals contain no electrons. The lowest unoccupied orbital or LUMO is π3 in 1,3-butadiene (or any simple conjugated diene).
|
 |
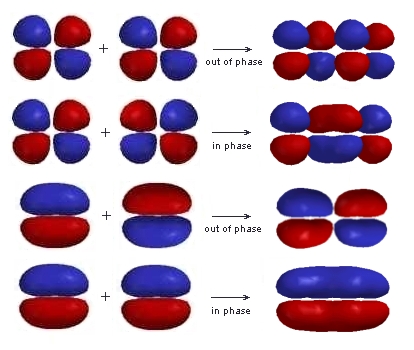
The relative energies of these orbitals can be accounted for by counting the number of bonding and anti-bonding interactions in each:
| © Dr. Ian Hunt, Department of Chemistry |