| Chapter 11 : Arenes and Aromaticity |
| Chapter 11 : Arenes and Aromaticity |
Qu: How can we recognise systems that are aromatic ?Based on the properties of aromatic compounds, there are FOUR criteria that are applied to the π system (not the molecule as a whole) that need to be met in order for the "special" aromatic stabilisation to be observed:Ans: By applying the criteria for aromaticity outlined below.
In order for a compound to be aromatic, all FOUR of these criteria must be met.
Study Tips: The Huckel rule....just think of "n" as an integer 0, 1, 2, 3... etc. it's just a number to feed into the simple formula to generate a set of numbers that correspond to the numbers of π electrons in aromatic systems : Those numbers are 2, 6, 10, 14 etc. π electrons (note that these numbers equate to 1, 3, 5, 7 etc. π electron pairs). So, if a cyclic, conjugated, planar π system contains one of these numbers of π electrons, then it's an aromatic system. For example, benzene is a 6 π electron system. "n" does not really relate to a simple structural property in the molecule. A simpler way to think of the Huckel rule is that it requires an odd number of π-electrons pairs. |
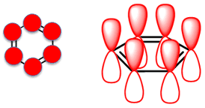
The most important and well known aromatic system is benzene. It consists of a set of 6 sp2 C arranged in a ring. This creates a set of 6 p orbitals, no gaps in the π system so we have a cyclic, conjugated π system. The planarity of the ring means that the interaction (orbital overlap) of the adjacent p orbitals is optimal. The 3 C=C in benzene mean that we have 3 pairs of π electrons = 6 π electrons = a 4n+2 number (a Huckel number) where n=1.
|
|
|
|
|
To aid in counting the electrons the following factors may help:
What about the terms "non-aromatic" and "anti-aromatic" ?
If a system satisfies all of the first three criteria (i.e. it has a cyclic, planar and conjugated π system), but has 4n π electrons in the cyclic conjugated π system (n = 0, 1, 2, 3 etc. which is equivalent to an even number of π-electrons pairs), then the system will be anti-aromatic. Note that anti-aromatic compounds are rare because they tend to be highly unstable.
If a system fails to satisfy at least one of the first three criteria (has a cyclic, planar and conjugated π system), then the system will be non-aromatic.
Similarly, systems with odd numbers of
π electrons in the cyclic conjugated π system (i.e. not 4n+2 or 4n systems) are typically non-aromatic.
| © Dr. Ian Hunt, Department of Chemistry |