| Chapter 11 : Arenes and Aromaticity |
| Chapter 11 : Arenes and Aromaticity |
Arenes and Aromaticity Answers
| Qu 1 | ||
| (a) AB is a neutral, 4 π-electron, anti-aromatic system. As B is in group III, it only has 6 electrons in the valence shell and hence is sp2 with an empty p orbital available for conjugation. AE could have worked as it is 4 π and anti-aromatic, but it is a cation. | 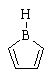 |
|
| (b) Pyridine A is a 6 π-electron, aromatic system. Pyridine is obviously related to benzene, with the lone pair on the N is in an sp2 orbital, perpendicular to the π system, and that the N is already involved in the π system in the C=N. The substituted furan, AC is also a 6 π-electron, aromatic system by virtue of one of the lone pairs on the sp2 O being in a p orbital. | 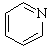 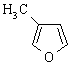 |
|
| (c) If n=2 in the Huckel rule, then we are after 10 π-electrons (so 5 pairs of electrons) which is satisfied by the outer loop in the interesting blue hydrocarbon azulene, BC. No other compounds have 10 π-electrons. | 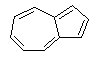 |
|
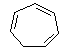
| (d) Cycloheptatriene is a non-aromatic, conjugated
6 π-electron system. Remember that conjugated only means interacting
π system so B is still conjugated, but it is not a cyclic conjugated
system required for aromaticity. |
 |
|
| (e) Non-conjugated implies that the the π systems are insulated from each other, probably by sp3 centers, such as those in D. | 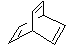 |
|
| (f) B non-aromatic as drawn and will give an
aromatic cation with a loss of a hydride ion. Looking at all the options, think about which molecules are non-aromatic as drawn: B, C, D, E, (not cyclic conjugated system) and AD. Removing an H- from B with give a 6 π aromatic cation (remember that carbocations are sp2 hybridised so there is an empty p orbital available as part of the π system. On loss of the hydride, C would be 4 π and anti-aromatic, D would still not be a conjugated cyclic system and E would be 2+ve. All the carbons in AD are already involved in a π system so losing a H- would not change that. |
 |
|
| (g) Use the list on non-aromatic compounds from part (f). For this type of question, you probably need to look for a system that has an exocyclic p bond, as in AD. The resonance of the carbonyl gives +C-O- which means we have a 2 p aromatic system based on the cyclopropenyl cation. | ||
| (h) Use the list of non-aromatic as drawn from part (f). C and E both have an aromatic conjugate base. This is similar to question 2(f), but now we remove a proton, H+, to get an anionic system with 2 more electrons, in both cases giving 6p electron systems. | 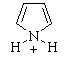 |
|
| Qu 2: | |
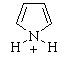 (a) The strongest acid is revealed by the
lowest pKa, so the conjugate acid of pyrrole, is the strongest
acid (a) The strongest acid is revealed by the
lowest pKa, so the conjugate acid of pyrrole, is the strongest
acid |
|
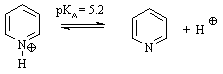 (b) At a pH 1 unit more acid than the pKa,
the relative ratio of pyridine, to its conjugate acid, is 1:10 is favour
of the protonated form. (b) At a pH 1 unit more acid than the pKa,
the relative ratio of pyridine, to its conjugate acid, is 1:10 is favour
of the protonated form. |
|
| (c) The pKa for the pyridine systems is 5.2, for aniline it is 4.6 So the aniline conjugate acid is a stronger acid, so the better base must be pyridine. In pyridine the lone pair is not delocalised (it is in fact perpendicular to the aromatic π system, but in aniline the N lone pair can be delocalised using resonance with the adjacent π system. | |
| (d) Pyrrole is a weaker base because while it is aromatic, its conjugate acid is non-aromatic. In comparison, pyridine and its conjugate acid are both aromatic. So protonation of pyrrole results in the loss of the aromatic stablisation. | |
| Qu 3: | |
| Resonance energy measures the extra stability of conjugated
systems compared to the same number of isolated C=C. Naphthalene (i) has a benzene ring plus an extra 2 conjugated C=C and so has a higher resonance energy than benzene (ii) itself (but not a great as two separated benzenes). Benzene is more aromatic and has a greater resonance energy than furan (iii). So i > ii > iii. |
|
| Qu 4: | |
| Option (i) is a vinyl carbocation (they are between primary and methyl cations is terms of stability) There is no resonance stabilisation here (try sketching the orbitals, the empty orbital associated with the +ve charge must be perpendicular to the C=C π system.. (ii) is a simple secondary carbocation. (iii) in a secondary benzylic system (so further resonance stability due to resonance with the aromatic π system is present). Thus the order is iii > ii > i. | |
| Qu 5: | Resonance energy measures the extra stability of a system compared to the same number of isolated double bonds. |
| In order to do calculate the resonance energy of anthracene,
we need to compare the experimental heat of hydrogenation (given in the
question as -116.2 kcal/mol) with that of isolated (non-interacting) double
bonds.
Since anthracene has 7 double bonds we get: 7 x -28.6 kcal/mol = -200.2 kcal/mol which is 84 kcal/mol more EXOTHERMIC than that of anthracene (compare -200.2 and -116.2) so we can conclude that anthracene is 84 kcal/mol more stable than 7 isolated double bonds. Resonance energy (anthracene) = 84 kcal/mol |
|
| (b) The Diels-Alder reaction to A gives a naphthalene
system (resonance energy = 61 kcal/mol) and that to B gives two benzenes (resonance energy = 2 x 36 kcal/mol = 72 kcal/mol) Therefore the greater stability of B compared to A drives the reaction (since two benzenes have greater resonance stability than a naphthalene) and despite the statistical effect (2 : 1 ways to get A rather than B) |
| © Dr. Ian Hunt, Department of Chemistry |