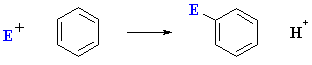 |
Chapter 12 : Reactions of Arenes. Electrophilic Aromatic
Substitution
|
 |
Electrophilic Aromatic Substitution
Overall an electrophilic aromatic susbtitution
(EArS) can be represented as follows:
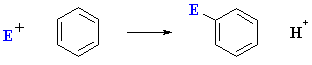
There are three fundamental components to an electrophilic
aromatic substitution mechanism:
- formation of the new σ bond from a C=C in the arene nucleophile
- removal of the proton by breaking the C-H σ bond
- reforming the C=C to restore the aromaticity
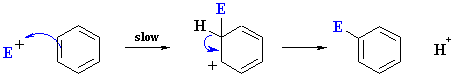
The mechanism is represented by the following series
of events:
- Formation of the reactive electrophile, E+ (not shown here) from the reagents
- Slow reaction of the arene C=C with the E+
to give a resonance stabilised carbocation
(see below)
- Loss of H+ from the carbocation
to restore the C=C and the aromatic system
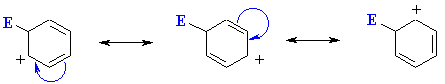 The reaction of the electrophile E+ with the arene is the slow step since it results
in the loss of aromaticity even though the resulting cation is still resonance
stablised. This carbocation is also described as the cyclohexdienyl
cation or arenium ion or
as a sigma-complex.
The reaction of the electrophile E+ with the arene is the slow step since it results
in the loss of aromaticity even though the resulting cation is still resonance
stablised. This carbocation is also described as the cyclohexdienyl
cation or arenium ion or
as a sigma-complex.