|
|
|
|
Nitration of Benzene
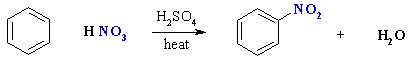
Summary
|
|
|
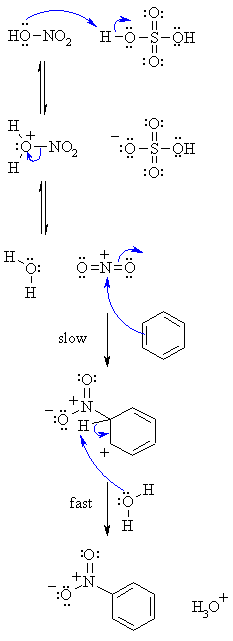
| Step 1: An acid / base reaction. Protonation of the hydroxy group of the nitric acid. This provides a better leaving group..... |
|
| Step 2: Loss of the leaving group, a water molecule provides the nitronium ion, the reactive electrophile. |
|
| Step 3: The electrophilic nitronium ion reacts with the nucleophilic C=C of the arene. This is the rate determining step as it destroys the aromaticity of the arene. |
|
| Step 4: Water functions as a base to remove the proton from the sp3 C bearing the nitro- group and reforms the C=C and the aromatic system. |
|
| © Dr. Ian Hunt, Department of Chemistry |