 |
|
 |
 |
|
 |
Making Polysubstituted Benzenes
Since the position of electrophilic attack on a substituted benzene ring is controlled by the substitutent already present rather than the approaching electrophile, the order of events in the synthesis of polysubstituted benzenes need careful planning to ensure success.
The two factors that need to be monitored are:
| Study Tip: Some students get confused over which group does the directing, the incoming electrophile, E+ , or the initial substituent, -X. Try thinking about it in terms of an aircraft (the E+ ) coming into land at an airport (the Ar-X )... it is the control tower at the airport on the ground (-X) that does the "directing" of which runway and which ramp the aircraft should go to) |
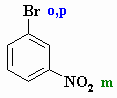 |
Here the nitro group
could direct to bromine to the correct position. Therefore in planning the synthesis we should use the nitro group to introduce the bromine |
PLANNED SYNTHESIS |
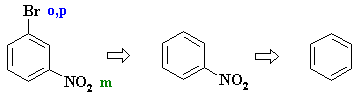
|
COMPLETE SYNTHESIS |
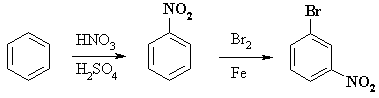
|
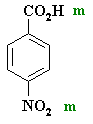
|
Here neither group
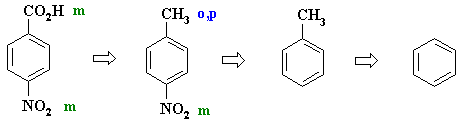
directs to the correct location of the other. But since the carboxylate could be introduced by the oxidation of a methyl group, which directs o,p- we can use the methyl to direct the nitro group to the correct position. |
PLANNED SYNTHESIS |
|
COMPLETE SYNTHESIS |
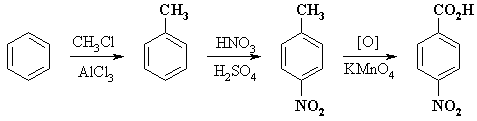
|
| |
Note that the oxidation has to be after the nitration to get the correct orientation and that the Friedel-Crafts alkylation has to before the nitration since nitrobenzene is too deactivated to undergo alkylation. |
| © Dr. Ian Hunt, Department of Chemistry |