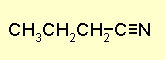PROBLEM #10
The following data is available from the question:
MS: M+ = ? g/mol. Lots of small peaks, not easy
to see if an M+ is visible, but at least 68g/mol
IR: There are no strong absorptions around 1715cm-1
for a C=O band, 3300cm-1 for O-H/N-H stretch, 1600cm-1
for C=C, or 1100cm-1 for C-O. There is an absorption at 2260cm-1
for a either CºC or CºN.
13C nmr: The proton decoupled spectrum shows 4 peaks indicating
4 types of C. By analysis of the chemical shifts, we have a deshielded
C at 120 ppm and a peaks at 20ppm, 19ppm and 13ppm that are probably hydrocarbon
portions.
1H nmr: First of all we have 3 types of H showing up.
After this, it's a good idea to tabulate the information to make sure you
get it all correctly matched up:
|
d/ppm
|
multiplicity
|
integration
|
Inference
|
|
2.3
|
triplet
|
2
|
slightly deshielded CH2 coupled to 2H |
|
1.7
|
sextet
|
2
|
probably a CH2 coupled to 5H |
|
1.1
|
triplet
|
3
|
CH3 coupled to 2H |
The coupling patterns for the -CH3 (1.1ppm, triplet) and
the -CH2- (2.3ppm, triplet) indicate that they are both connected
to -CH2- (1.7ppm, sextet) so we have an n-propyl group:
CH3CH2CH2- .
Summary....
The only functional group indicated in the IR showed is a CºC
or CºN.
13C peak at 120ppm suggests that this is a CºN
(usually 110-125ppm) rather than CºC (usually
70-90ppm).
H nmr gave us an n-propyl chain =
So with this information we have the following pieces: CH3CH2CH2-
and -CºN.
Use this to check the molecular formula so far : C4H7N
= 4 x 12 + 7 x 1 + 14 = 69 g/mol
This is at consistent with the minimum MW from the mass spectrum.
Altogether...
With the pieces we have : CH3CH2CH2-
and
-CºN.
This means we only have the 2 end groups which can only be out together
in one way.
Note the nitrile is supported by the IR CºN
stretch and 13C chemical shift. H nmr gives the propyl chain. The nitrile
group slightly deshields the attached methylene. |
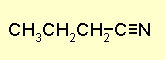
butyronitrile or
n-propyl cyanide
|
The final step should always be to check what you have drawn.
The easiest thing to check is usually the coupling patterns you would
expect to see, and the chemical shifts of each unit.
You should be asking yourself : "Does my answer give me what the
H-nmr shows ?"