| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
Different types of Hydrogen
The issues to understand from this page are:
This may seem a trivial concept, but the idea of establishing the number of kinds of hydrogens (or other atoms such as C) is a very important useful concept, especially in NMR spectroscopy where for example, different types of H (or other atoms) typically each give rise to a different signal. The idea is developed below for H atoms, but can be extended to other types of atom.
What do we mean?
A hydrogen atom is "different" to another hydrogen atom if
it is not in an identical environment (location) to the other hydrogen. This could mean
it is attached to a different type of atom (e.g. compare CH vs OH, or sp3 CH vs sp2 CH),
or due to the number of adjacent H (e.g. CH3- vs -CH2-
) or just at a different point in a chain (e.g. compare the H in the methylene
(CH2) groups in CH3CH2CH2OH which has 4 types of H.
Counting
different types of Hydrogen
There are three methods that can be used to count the number of kinds
of H (each achieve the same result). You will probably find it best to
master the first of these methods and over time you will find yourself moving
towards the 3rd method)
For example:
Qu : If
you remove one H atom from chloroethane and substitute it for another Cl atom,
how many different molecules can you make ?
Ans : Two products, 1,1-dichloroethane
and 1,2-dichloroethane. Hence there are two types of H in chloroethane
2. Verbal description
The verbal method requires that you describe the position of each H within the
molecule. If you need to use different words to describe two H atoms,
then they represent different types of H.
For example:
|
|
|
QUESTIONS
|
|
|
|
|
|
|
|
|
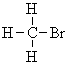 ANSWER |
| Homotopic | Replacement of the groups gives the same product | For example, it doesn't matter which of the H atoms in bromomethane is replaced with chlorine, we always get bromochloromethane. Hence these three H are said to be homotopic. | Homotopic H always give the same NMR signal. |
| Enantiotopic | Replacement of the groups gives enantiomers |
Consider the H atoms in the methylene
group in bromoethane. If we replace one of those H with a Cl, we create a chirality center. Therefore
depending on which of the two H
is replaced, we get one enantiomer or the other. Hence these two H are said to be enantiotopic.
|
Enantiotopic H give the same NMR signal in an achiral environment (normal NMR conditions) |
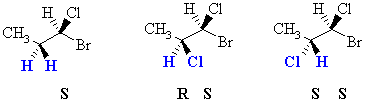
| Diastereotopic | Replacement of the groups gives diastereomers. | Consider the H atoms in the methylene
group in 1-bromo-1-chloropropane. There is already a chirality center at
C1. If we replace one of those H
with a Cl, we create a new chirality center. Therefore depending
on which of the two H is replaced, we get one diastereomer or the other.
Hence these two H are said to be diastereotopic.
|
Diastereotopic H : are different and in principle they give rise to different signals and hence they can couple to each other (see later). |
QUESTIONS
| © Dr. Ian Hunt, Department of Chemistry |