| Chapter 13: Spectroscopy |
| Chapter 13: Spectroscopy |
The Aromatic Region
In the context of this page, the term "aromatic hydrogens" typically means the H atoms attached to a simple benzene ring. The typical region of the H-NMR for these aromatic H atoms is between about 6.5 and 8.0 ppm.
The appearence of the aromatic region can vary depending on 3 important factors (1) the number of substituents attached to the benzene ring and (2) the nature of those substituents and (3) the relative positions of those substitents. Note that as the substitution pattern changes the number of types of aromatic H may change.
The integration of the aromatic region is a potentially simple way to determine the number of substituents attached to the benzene ring simply by subtracting the number of aromatic H from 6, e.g. if you observe 4 aromatic H, then we must have 2 other substituents. This simple idea is often overlooked.
A series of H-NMR spectra of simple, monosubsituted benzenes are shown below, along with the H-NMR of benzene for comparison. Note that a monosubstituted benzene would typically have 3 types of aromatic H on that benzene ring.
Two things are apparent:
The key message is that the appearence of the aromatic region changes depending on the nature of the substitution so don't read too much into that initially. First, use the integration to determine the number of aromatic H and hence the number of substituents that are present. If there is more than one substitutent, then you will need to determine the substitution pattern where the number of types of aromatic C or H and the H NMR coupling of the aromatic region might provide useful information.
Benzene
|
Only 1 type of aromatic H, chemical shift 7.26 ppm |
Toluene
|
Since the alkyl group (R-) is only a weak electron donor, the electron density on the aromatic ring is not changed much. Therefore, while there a 3 types of ArH, the H are in very similar environments : accidental equivalence in the aromatic region. |
Anisole
|
An alkoxy group (RO-) is a strong electron donor (via resonance) and this adds electron density to the aromatic ring resulting in a shielding effect and a lower chemical shift especially in the ortho and para positions. |
Nitrobenzene
|
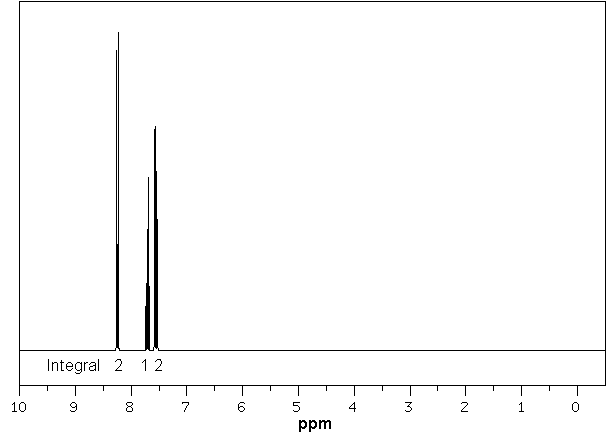 A nitro group (NO2-) is a strong electron withdrawing group (via resonance and induction) and this removes electron density to the aromatic ring resulting in a deshielding effect (increased chemical shift). |
| © Dr. Ian Hunt, Department of Chemistry |