| Chapter 14: Organometallic Compounds |
| Chapter 14: Organometallic Compounds |
Organometallic Compounds
Nomenclature:
Organometallic compounds are normally named as substituted metals, e.g.
alkyl metal or alkyl metal halide.
Organomagnesium compounds are generally referred to as Grignard reagents.
Examples: CH3Li = methyllithium, CH3MgBr = methylmagnesium bromide.
| H 2.1 |
He | ||||||||||||||||
| Li 1.0 |
Be 1.5 |
B 2.0 |
C 2.5 |
N 3.0 |
O 3.5 |
F 4.0 |
Ne | ||||||||||
| Na 0.9 |
Mg 1.2 |
Al |
Si 1.8 |
P 2.1 |
S 2.5 |
Cl 3.0 |
Ar | ||||||||||
| K 0.8 |
Ca 1.0 |
Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br 2.8 |
Kr |
 |
 |
 |
|
methyl
chloride
|
methyllithium
|
methylmagnesium bromide
|
|
CH3Cl
|
CH3Li
|
CH3MgBr
|
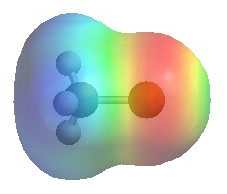
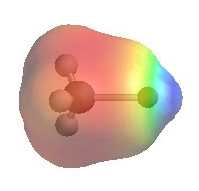
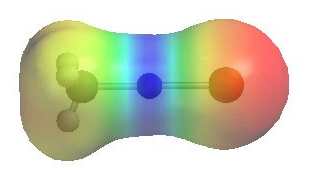
| The images show
the electrostatic potentials for methyl chloride, methyl lithium and methyl
magnesium bromide. The more red an area is, the higher the electron density and the more blue an area is, the lower the electron density.
It is reasonable to think of these organometallic compounds as R- M+ QUESTION Can you think of other types of anions where this "dual" reactivity is also very important ? ANSWER |
||
| The following equation represents the loss of a proton from a generic hydrocarbon forming a carbanion: | 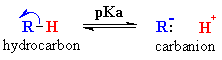 |
|
The table shows the pKa's
of a selection of representative systems. Note that the hydrocarbons are
very weak acids, implying that the carbanions will be strong bases.
QUESTION Explain the pKa order: ethane > ethene > ethyne ANSWER |
| © Dr. Ian Hunt, Department of Chemistry |