| Chapter 15: Alcohols, Diols and Thiols |
| Chapter 15: Alcohols, Diols and Thiols |
Hydroxylation of Alkenes
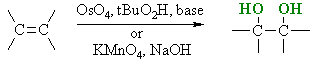
Reaction Type : Electrophilic Addition
Summary
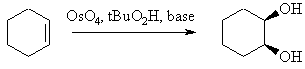 |
QUESTIONS
What other reaction is commonly used as a functional group test for alkenes
? ANSWER
What is the oxidation state of the osmium in osmium tetroxide ? ANSWER
Is the osmium oxidised or reduced during the formation of the osmate ester ? ANSWER
What is the oxidation state of the potassium permanganate ? ANSWER
Related reactions
|
|
|
| Step 1:
The p electrons in the alkene act as a nucleophile forming a favourable 5 membered ring as a cyclic osmate ester as an intermediate. Note the origin of the cis stereochemistry. |
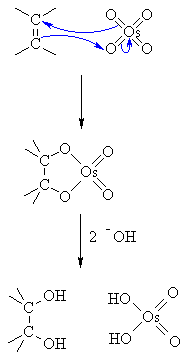
|
| Step 2:
The hydroxide liberates the cis-diol and the reduced osmium species.... this would then be reoxidised by the peroxide co-oxidant. |
|
| © Dr. Ian Hunt, Department of Chemistry |