| Chapter 15: Alcohols, Diols and Thiols |
| Chapter 15: Alcohols, Diols and Thiols |
Oxidative Cleavage of Diols
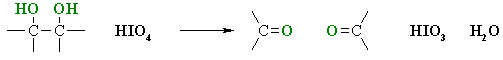
Reaction type: Oxidation-reduction
Summary
|
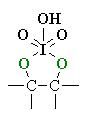 |
| PERIODATE
CLEAVAGE OF 1,2-DIOLS |
|
| The mechanism is not trivial, so attention here is focussed on the actual cleavage step. Prior to this, the alcohol reacts to form a cyclic periodate ester (shown). The periodate ester undergoes arearrangement of the electrons, cleaving the C-C bond, and forming two C=O. | 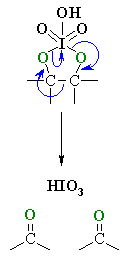
|
| © Dr. Ian Hunt, Department of Chemistry |