| Qu 1: |
(a) Since 1-propanol
would be the anti-Markovnikov alcohol, use BH3 then H2O2
/ NaOH
|
|
(b) Need to add
a C, so open epoxide with an organometallic reagent, e.g. CH3MgBr
in Et2O or THF, followed by an acidic work-up.
|
|
(c) Right number
of C, so simple reduction using either LiAlH4 in THF then acidic
work-up or NaBH4 in EtOH
|
|
(d) Right number
of C, so simple reduction using LiAlH4 in THF then acidic work-up.
NaBH4 will not reduce RCO2H
|
|
(e) Reduction
of a propanoate ester with LiAlH4 in THF will give the primary
alcohol.
|
|
|
| Qu 2: |
(a) Since 2-propanol
would be the Markovnikov alcohol, use aq. H2SO4
/ heat.
|
|
(b) Right number
of C, so simple reduction using either LiAlH4 in THF then acidic
work-up or NaBH4 in EtOH
|
|
(c) Need to add
a C, use an organometallic reagent, e.g. CH3MgBr in
Et2O or THF, followed by an acidic work-up.
|
|
(d) Right number
of C, just need to change the functional group. Use aq. NaOH.
|
|
|
| Qu 3: |
(a) cis-1,2-cyclohexanediol
|
|
(b) trans-1,2-cyclohexanediol
|
|
(c) (R,R)- and
(S,S)-2,3-butanediol
|
|
(d) meso-2,3-butanediol
|
|
(e) (R,R)- and
(S,S)-2,3-butanediol. Compare this to reaction (d), changing the stereochemistry
of the overall additon process to anti means that the products will be
diastereomeric with those in (d).
|
|
(f) They are diastereomers.
Since the addition is syn, the relationship is defined by the relationship
of the alkene starting materials.
|
|
|
| Qu 4: |
(a) Thionyl chloride
converts ROH to RCl, so benzyl chloride, C6H5CH2Cl.
|
|
(b) A Williamson
ether synthesis, giving C6H5CH2OCH2CH3
|
|
(c) Under aqueous,
the product will be benzoic acid, C6H5CO2H
|
|
(d) With the more
selective oxidation conditions, the product will be benzaldehyde, C6H5CHO
|
|
(e) An ester preparation
giving benzyl ethanoate, CH3CO2CH2C6H5
|
|
|
| Qu 5: |
In each case,
disconnect the alcoholic portion from the acid portion:
|
|
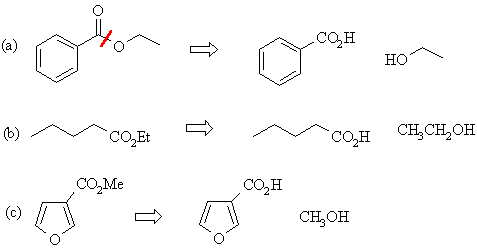
|
|
|
| Qu 6: |
Periodic acid,
HIO4, causes oxidative cleavage of 1,2-diols.
|
| |
In the case of
a cyclic diol, this would break the ring. The product here would
be 1,6-hexanedial. 
|
|
|
| Qu 7: |
The best method
for ether synthesis is the Williamson method from an alcohol and an alkyl
halide.
|
|
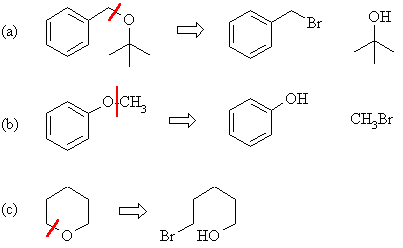 |
(a) This is the best
disconnection to a primary halide (good SN2) and the
tertiary alcohol. The tertiary bromide would be prone to elimination. |
| (b) This is the only
choice since aryl bromides do not undergo substitution, we must
make it the alcoholic component. |
| (c) A symmetrical cyclic
ether so we can disconnect either C-O bond, it makes no difference. |
|

