| Chapter 16: Ethers, Epoxides and Sulfides |
| Chapter 16: Ethers, Epoxides and Sulfides |
| Qu1: | Ethers are cleaved by strong acids like HBr and HI to give an alcohol and an alkyl halide. |
| However, in the
presence of excess HI, alcohols react to give alkyl iodides. (a) 2 moles CH3CH2I (b) (CH3)3CI and CH3I (c) I-CH2CH2CH2CH2-I (d) I-CH2CH2-I |
|
| |
|
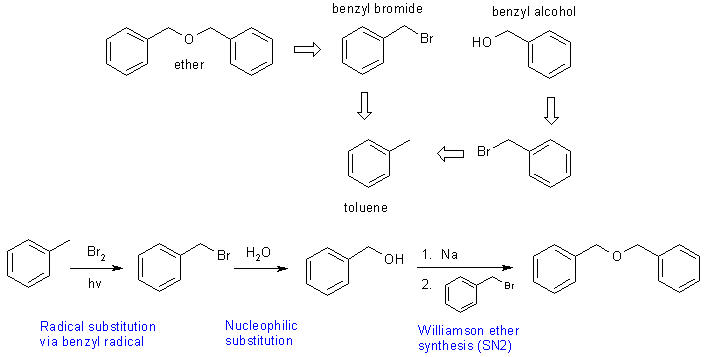
| Qu2: | The challenge is the synthesis of the ether from toluene. First question, how do we make ethers ? |
A Williamson
ether synthesis requires and alkoxide and an alkyl halide. In this case,
it is necessary to make the alcohol from the halide by a simple substitution
since the halide can be obtained from the toluene via a radical subtituion.
The retrosynthesis and the synthesis are shown below:
|
|
| |
|
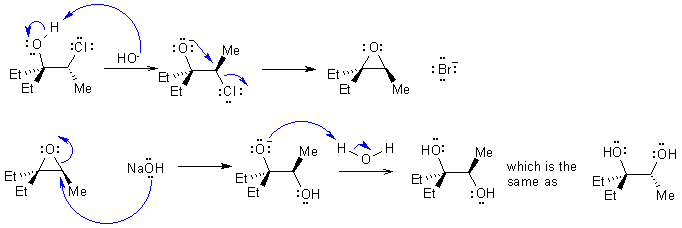
| Qu3: | In an SN2 process we normally get inversion, and in SN1 racemisation. |
Since we don't
have racemisation it can't be SN1, so it must be SN2.
In order to get overall retention, this could indicate 2 sequential SN2
reactions. The process is that the 1,2-halohydrin forms an epoxide via
an intramolecular SN2 displacement of chloride, followed by
ring opening of the epoxide under basic conditions via another SN2
reaction.
|
|