|
|
|
|
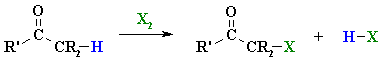
Summary
|
|
|
| Step 1: First, tautomerise the carbonyl to its enol tautomer (mechanism not shown here : review ?) |
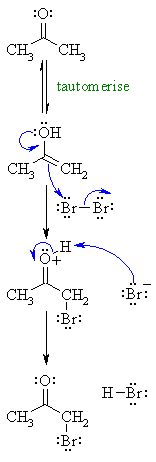 |
| Step 2: Here the electrons from the oxygen are used as they enhance the nucleophilicity of the alkene, as a result we end up with an oxonium ion and a bromide ion. |
|
| Step 3: An acid / base reaction. Here the bromide ion is used to deprotonate the oxonium ion. |
|
| © Dr. Ian Hunt, Department of Chemistry |