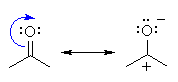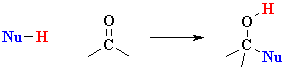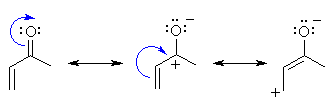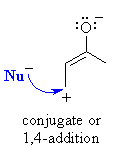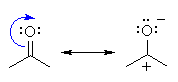
|
The reactivity of simple aldehydes
and ketones can be rationalised by looking at the resonance contributors
(see left). |
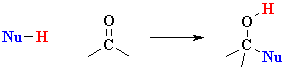 |
You should recall
that they are attacked by nucleophiles at the C=O due to the electrophilic
character of the carbonyl C. |
|
|
|
|
2-propenal
(acrolein)
|
3-butenone
(methyl vinyl ketone)
|
1,3-butadiene
|
|
In the JMOL images to the
left, are examples of a conjugated aldehyde and a conjugated ketone and
a conjugated diene for comparison.
In each compound note the geometric relationship between the 2 double
bonds they all lie in the same plane.
This means the the π-orbitals of the two double
bond systems are aligned and there is an interaction between them... RESONANCE....
this "modifies" the reactivity to some degree. |
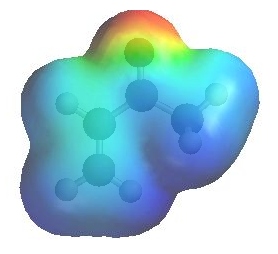 Electrostatic potential of 3-butenone
Electrostatic potential of 3-butenone
|
Look at the electrostatic
potential diagram of the ketone (left). Note the red region show
the high electron density at the O of the C=O and the lower
electron density of the conjugated C=C, compare this with
the electrostatic potential diagram of the diene to the right....
here the C=C are electron rich. |
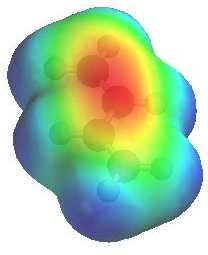 Electrostatic potential of 1,3-butadiene
Electrostatic potential of 1,3-butadiene |
|
|
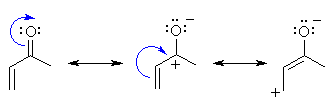 |
In a conjugated
carbonyl system, there is an extra resonance structure that also shows
electrophilic character at the terminal alkene
C. Compare these resonance contributors with the electrostatic potential
of 3-butenone shown above.... they tell us the same thing. |
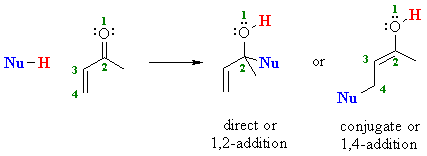
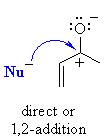
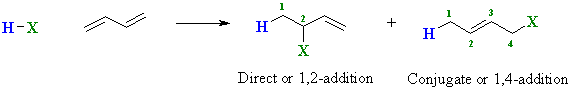
| Therefore, αβ-unsaturated
aldehydes and ketones can potentially react with nucleophiles at two sites
: directly at the carbonyl C or the end of the conjugated system: |