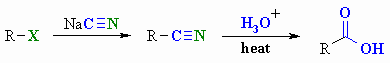 |
Chapter 19:
Carboxylic Acids |
 |
Hydrolysis of Nitriles
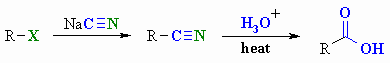
Reaction type : Nucleophilic Substitution
then Nucleophilic Addition then Nucleophilic Acyl Substitution
!
Summary
- 1o and 2o alkyl
halides (X = Cl, Br, I) or tosylates
undergo SN2 substitution with
cyanide salts to give nitriles.
- Nitriles can be hydrolysed to
carboxylic acids without the isolation of the amide intermediate.
- Note that the carbon skeleton
is extended by 1 C atom during this reaction sequence.
- Although aromatic nitriles cannot
be prepared via the SN2 reaction, they too can be converted to
the aromatic carboxylic acid by hydrolysis.