| Chapter 19: Carboxylic Acids |
| Chapter 19: Carboxylic Acids |
| Qu1: | (i) increasing acidity : |
| CH3CH2OH
< CH3CH2SH < CH3CO2H
< CCl3CO2H Resonance
stabilisation in carboxylates makes carboxylic acids more acidic than
alcohols or thiols. Electron withdrawing groups, here -Cl, increase the acidity due to further stabilisation of the carboxylate. Thiols are more acidic than alcohols due to weaker X-H bond and the ability of S to accommodate extra electrons (size). |
|
| (ii) increasing pKa : | |
| CCl3CO2H
< CH3CO2H < CH3CH2SH
< CH3CH2OH Remember
the lower the pKa the stronger the acid, so once you have part
(i), this is just be the opposite. Tip: When asked about pKa trends, it may be easier to think in terms of acidity then remember to flip the order. |
|
| Qu 2: | Note the identical nature of the reactions that the aliphatic and aromatic acid undergo: |
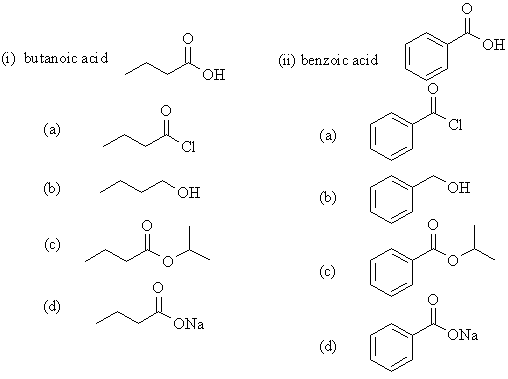
(a) Thionyl chloride, SOCl2,
is used to prepare acyl chlorides, the base removes the HCl by-product.
|
|
| Qu 3: | First, note that we have an homologous series of C3 to C6 acids we are trying to make. |
| Here is a scheme
collecting possible syntheses together (based on the more important reactions)
|
|
| (a) Propanoic
acid : need to loose a C from C4. We can do this via ozonolysis of
an alkene, which we can obtain by eliminating the alkyl halide.
(b) Butanoic acid : oxidation of the corresponding alcohol will give the carboxylic acid, so prepare the alcohol by substitution. (c) Pentanoic acid : need to gain a C to get C5. One way to do this is via the reaction of the Grignard reagent with carbon dioxide. Alternatively, substitution with NaCN then hydrolysis would also work. (d) Hexanoic acid : need to gain 2C to get C6. Reaction of the Grignard reagent with ethylene oxide gets the right number of C and a primary alcohol ready for oxidation to the acid. |