| Chapter 22: Amines |
| Chapter 22: Amines |
Selectivity of the Hofmann Elimination
In general E2 reactions occur most
rapidly when the H-C bond and C-LG
bonds at 180o with respect to each other. This is described as an
antiperiplanar conformation. This conformation positions the σ
bonds that are being broken in the correct alignment to become the p bond.
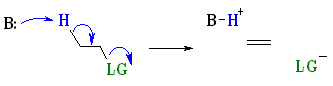
|
||
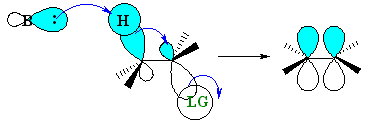 |
The staggered, antiperiplanar alignment is preferred because it aligns the two σbonds that become the π bond. | 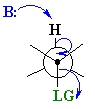 |
Lets' look at the Hofmann elimination and the selectivity of the reaction:
|
|
The JMOL image to
the left shows the calculated energy minima of the sec-butyltrimethylammonium
compound.
Since these reactions are E2, we need to look at the bonds that are antiperiplanar to the C-N bond that is broken. Orient the molecule so that you can see this. The only C-H bond antiperiplanar
to the C-N bond is in the C1 methyl group and so leads to the formation
of 1-butene. |
|
|
The JMOL image
to the left shows the of the sec-butyltrimethylammonium compound
in the conformation that leads to the formation of trans-2-butene.
Again look at the bonds that are antiperiplanar to the C-N bond that is broken, this time there are two. Can you see them ? One is on the -CH2- and leads to the formation of 2-butene, the other is in the C1 methyl group and so leads to the formation of 1-butene. Note that this conformation
puts the large N(CH3)3 and the C4 methyl group
gauche to each other, this destabilises the conformation
compared to the one that leads to 1-butene. Using the space filling
model to check..... |
| © Dr. Ian Hunt, Department of Chemistry |