| Chapter 23: Aryl Halides |
Addition Reactions of Benzyne
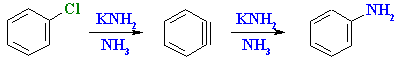
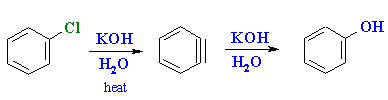
Summary
- The strained triple bond in benzyne makes it reactive towards addition reactions.
- Additions of H2O and NH3 are commonly encountered are a result of the methods of formation (see above).
- Substituted benzynes can also be formed, where the subsequent addition reaction typically gives mixtures of products:
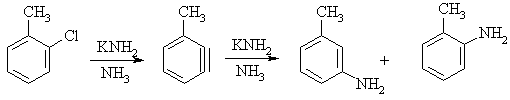
- Diels-Alder reactions of Benzyne
- Addition reactions of Alkynes and Alkenes
|
|
|
| Step 1:
The N in amide functions as the nucleophile and attacks the reactive triple bond C in benzyne creating the new C-N bond and an intermediate carbanion. |
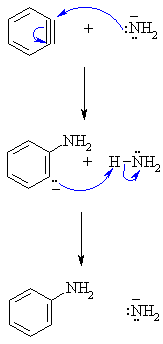 |
| Step 2:
An acid/base reaction. Rapid protonation of the reactive carbanion from the ammonia forms the aniline and anotheer molecule of the amide ion. |
|
| © Dr. Ian Hunt, Department of Chemistry |