|
|
|
|
| Qu1: | This is a Fischer projection. |
|||||||||
| Qu 2: | In a Fischer projection the horizontal bonds are towards you, the vertical away as shown below: | |||||||||
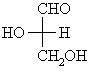 = = 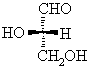 HO > CH=O > CH2OH > H. Now with the lowest priority group away, the sense of rotation of highest to lowest priority is counter-clockwise, so this is the S stereoisomer. Review this ? To assign the D / L configuration, need to remember that in the Fischer projection the -OH group is on the left in the L stereoisomer. |
||||||||||
| Qu 3: | This is the mirror image of that in qu2, so this is the R- or D-glyceraldehyde. | |||||||||
| Note how important it is to remember that the Fischer diagram of sugars needs to be drawn with the carbonyl (oxidised) end uppermost. | ||||||||||
| Qu 4:
|
Both are Fischer projections, drawn the same way up, so a straight comparison can be made. The systems are isomers, differing only in the arrangement of the groups at one of the two chirality centers. So they are stereoisomers, specifically diastereomers. | |||||||||
| Qu 5: | In each case, the anomeric center has been indicated by * . It is important that you can recognise this center... you need it for part (ii) | |||||||||
|
||||||||||
| Qu 6: | In each case the anomeric center / carbonyl group has been indicated by * . It is important that you can recognise this center... you need it for part (i) | |||||||||
|
||||||||||
| Qu 7: | A reducing sugar needs to have an aldehyde, a hemi-acetal group or be a ketose that can tautomerise to a aldose. | |||||||||
| (a) is not a reducing sugar as there is not an -OH at the anomeric
center.
(b) Sucrose is not a reducing sugar as both anomeric centers are glycosides (i.e. acetals) not hemi-acetals. (c) Glucose is a reducing sugar, here the aldehyde group is obvious. |