- Functional group is an alkene, therefore suffix = -ene
- There are two alkenes, so insert the multiplier di
- The longest continuous chain is C4 therefore root
plus "a" = buta
- The C=C units are both at the ends so we can number
from either end
- Locants for C=C units are therefore 1- and 3-
1,3-butadiene
or
buta-1,3-diene
|

CH2=CHH=CH2 |
- Functional group is an alkene, therefore suffix = -ene
- There are two alkenes, so insert the multiplier di
- The longest continuous chain is C5 therefore root plus
"a" = penta
- The first point of difference requires that we
number from the right as
drawn
- Locants for C=C units are therefore 1- and 3-
- The C3=C4 alkene has E stereochemistry
(E)-1,3-pentadiene
or
(E)-penta-1,3-diene
|

CH3CH=CHH=CH2
|
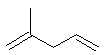
- Functional group is an alkene, therefore suffix = -ene
- There are two alkenes, so insert the multiplier di
- The longest continuous chain is C5 therefore root plus
"a" = penta
- The substituent is a C1 alkyl group i.e. a methyl group
- The first point of difference doesn't distinguish
the C=C
- So, need to apply the first point of
difference to the alkyl substituent.
- The first point of difference requires that we
number from the left as drawn
- The methyl group locant is 2-
- Therefore the locants for C=C units are 1- and 4-
2-methyl-1,4-pentadiene
or
2-methylpenta-1,4-diene
|
CH3C(CH3)=CHH=CH2
|