 |
 |
The IUPAC name of an organic
molecule is assembled from components that describe various features of the
molecule.
Functional group suffix
This is added to the end of the name based on the principal functional
group.
Root
This defines the number of atoms (usually carbon atoms) in the longest continuous
chain that contains the principal functional group.
Substituent prefix
Any groups other than the principal functional group are substituents
and are added to the beginning of the name in alphabetical order.
Multiplier
If a group occurs more than once, a simple multiplier (e.g. di, tri, tetra, etc.) is used to indicate how many
times it occurs.
Locants
Locants are numbers (or occasionally letters) that define the position
of the principal functional group and substituents. Typically
there needs to be a locant for each functional groups and each substituent.
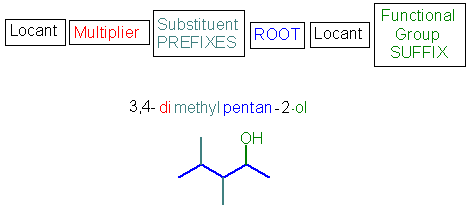
The basic structure of the IUPAC name is shown schematically below :
 |
|
| Let's work through the example shown - we will expand on the details in the pages ahead. | |
|
|
|
|
|
|
|
|
|
|
|
hence : 3,4-dimethylpentan-2-ol Note : the pentan-2-ol could also be written as 2-pentanol |
| ©Dr. Ian Hunt, Department of Chemistry |