- Functional group is an alcohol, therefore suffix = -ol
- Hydrocarbon structure is an alkane therefore -ane
- There are two alcohols, so insert the multiplier di
- The longest continuous chain is C2 therefore root = eth
- Locants for -OH units are 1- and 2-
ethane-1,2-diol
or
1,2-ethanediol
|

HOCH2CH2OH |
|
- Functional group is an alcohol, therefore suffix = -ol
- Hydrocarbon structure is an alkane therefore -ane
- There are two alcohols, so insert the multiplier di
- The longest continuous chain is C3 therefore root = prop
- Locants for -OH units are 1- and 2-
propane-1,2-diol
or
1,2-propanediol
|
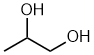
CH3CH(OH)CH2OH
|
|
- Functional group is an alcohol, therefore suffix = -ol
- Hydrocarbon structure is an alkane therefore -ane
- There are two alcohols, so insert the multiplier di
- The longest continuous chain is C4 therefore root = but
- Locants for -OH units are 1- and 4-
butane-1,4-diol
or
1,4-butanediol
|
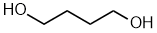
HOCH2CH2CH2CH2OH |
|
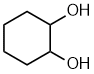
- Functional group is an alcohol, therefore suffix = -ol
- Hydrocarbon structure is an alkane therefore -ane
- There are two alcohols, so insert the multiplier di
- The ring is C6 therefore root = cyclohex
- Locants for -OH units are 1- and 2-
cyclohexane-1,2-diol
or
1,2-cyclohexanediol
|
|
|