 |
|
 |
|
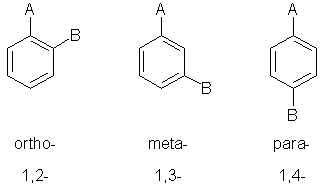
Numerical locants method:
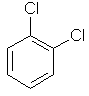
1,2-dichlorobenzene
|
 |
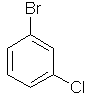
1-bromo-3-chlorobenzene
|
 |
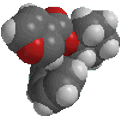
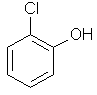
2-chlorophenol
|
 |
Ortho / meta / para method:
ortho-dichlorobenzene
or o-dichlorobenzene |
 |
meta-bromochlorobenzene
or m-bromochlorobenzene |
 |
ortho-chlorophenol
or o-chlorophenol |
 |
| ©Dr. Ian Hunt, Department of Chemistry |