Alkene
oxide style:
- Functional group is an epoxide, therefore suffix = -ene oxide
- The longest continuous chain is C3 therefore root = prop
- Location of "alkene" is unambiguous, so no locant needed.
propene oxide
Epoxy style:
- The longest continuous chain is C3 therefore root = prop
- The epoxide is a substituent therefore prefix = epoxy
- Number to give epoxide (only group present) the lowest locants
= 1,2-
1,2-epoxypropane
|
|
|
Alkene
oxide style:
- Functional group is an epoxide, therefore suffix = -ene oxide
- The longest continuous chain is C6 therefore root = hex
- The system is cyclic therefore prefix = cyclo
- Location of "alkene" is unambiguous, so no locant needed.
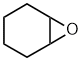
cyclohexene oxide
Epoxy style:
- The longest continuous chain is C6 therefore root = hex
- The root system is cyclic therefore prefix = cyclo
- The epoxide is a substituent therefore prefix = epoxy
- Number to give epoxide (only group present) the lowest locants
= 1,2-
1,2-epoxycyclohexane
|
 |
|
Alkene
oxide style:
- Functional group is an epoxide, therefore suffix = -ene oxide
- The longest continuous chain is C6 therefore root = hex
- There is a C1 alkyl substituent = methyl
- The first point of difference rule requires numbering from the right
as drawn to make the "alkene" locant = 2-
- Hence the methyl group locant = 5-
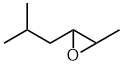
5-methyl-2-hexene oxide
Epoxy style:
- The longest continuous chain is C6 therefore root = hex
- There is a C1 alkyl substituent = methyl
- The first point of difference rule requires numbering from the right
as drawn
- The epoxide is a substituent therefore prefix = epoxy
- Number to give epoxide (only group present) the lowest locants =
2,3-
2,3-epoxy-5-methylhexane
|
 |
|