 |
Basic IUPAC Organic Nomenclature |
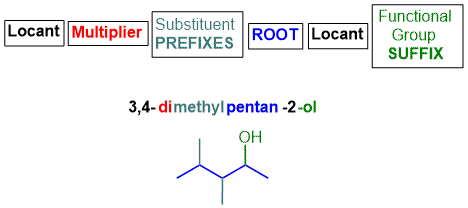
What's in a name?
The IUPAC name of an organic
molecule is assembled from components that describe various features and parts of the
molecule.
Functional group suffix
There can be several parts to this, (a) the first part depends on the hydrocarbon functional groups, and (b) the second part depends on the other functional groups (if any):
(a) the suffix(es) for hydrocarbon functional groups alkenes and / or alkynes (if any) are included after the "root" (see below),
(b)
the suffix for the principal functional
group is added to the end of the name.
Root
This defines the number of atoms (usually carbon atoms) in the longest continuous
chain that contains the principal functional group.
Substituent prefix
Any groups other than the principal functional group appended to the root chain are called substituents, i.e. they have replaced an H atom on that root chain.
Substituents are added to the beginning of the name and are listed in alphabetical order.
Multiplier
If a functional group or substituent occurs more than once, a simple multiplier (e.g. di, tri, tetra, etc.) is used to indicate how many
times it occurs.
Locants
Locants are numbers (or occasionally letters e.g. N-) that define
the positions of the functional group(s) and substituents.
Typically, there needs to be a locant for each functional group and each substituent.
The 1993 modifications requires that the locant for the principal functional
group is placed before the functional group suffix, e.g. pentan-2-ol,
see below.
Note that the functional group priority order (see functional group page) only applies if the functional group is being named as the principal functional group (i.e. it contributes as the functional group suffix), and the functional group priority order does not apply if the group is being named as a substituent.
The basic structure of the IUPAC name is shown schematically below :