 |
Basic IUPAC Organic Nomenclature
|
 |
Basic IUPAC Organic Nomenclature
|
Stereochemistry
Stereoisomers are molecules that have the same connectivity (i.e. the same pieces in the same order) but differ only in the arrangement of those pieces in space.
For
example, but-2-ene, CH3CH=CHCH3, has two possible stereoisomers due to the relative spatial positions of the -H and -CH3 groups on each end of the C=C unit (where there is restricted rotation).
| methyl groups on the same side | 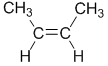 |
||
| methyl groups on opposite side | 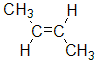 |
 |
Since these types of isomers are different molecules with different properties, they need to have individual and unique names.
The E- / Z- (alkenes) and R- / S- (chirality centers) nomenclature methods for naming stereoisomers are based on the use of the Cahn-Ingold-Prelog priority rules used for naming stereocenters.
These rules are used to establish the priority of the groups attached to the stereocenter and are based on atomic number, and the first point of difference. The rules are outlined below, and specific examples are provided within the following pages for the different scenarios, see the links below.
Cahn-Ingold-Prelog priority rules
In order to rank the groups at a stereocenter (e.g. an alkene or a chirality center):
(1) identify the stereocenter (it's a good idea to label it say with "*")
(2) identify
each of the atoms attached to that stereocenter
(3) assign
the priority of the atom / group based on the atomic number of that atom. Higher atomic number = higher priority.
If the same type of atom is connected, then we need to look at the next atoms in the groups / chain moving away from the stereocenter until we find the first point of difference. A good method for doing this is to write down the attached atoms and then if needed, a list of the atoms they are attached to in priority order (i.e. atomic number)
Once the difference has been identified, the relative priorites can be determined.If a multiple bond is encountered, treat it as if the atoms are attached by the same number of single bonds e.g. C=C is treated as 2 x C-C and C=O is 2 x C-O
| Study tip for applying the Cahn-Ingold-Prelog priority rules (1) Label the stereocenters with * (2) List out the attached atoms in priority order |
Two areas of stereochemistry are described in the following pages:
(1) stereoisomerism at alkenes (e.g. cis- / trans- or E- / Z-)
(2) stereoisomerism at chirality centers (e.g.
R- / S-)
| ©Dr. Ian Hunt, Department of Chemistry |