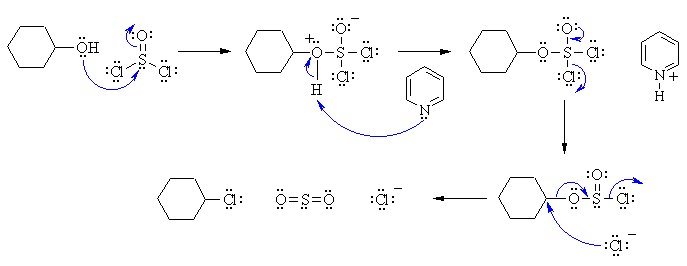
I This reaction converts an alcohol into an alkyl chloride, usually via an SN2 type mechanism. Remember -OH is a poor leaving group. Here the O becomes attached to the S and eventually leaves as part of sulfur dioxide, SO2 when the chloride ion attacks as a nucleophile.

|
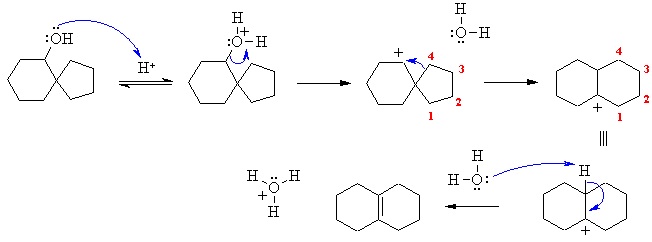
This is based on the substitution experiment and the theories behind SN1 and SN2 reactions. First challenge is to draw the structures correctly (see right). Both systems are alkyl halides with reasonable leaving groups (halide ions). |
 |
|
In SN2, where the Nu attacks and displaces the leaving group and the same time. The key factor is steric hindrance. Since both these systems are primary, then the approach of the Nu is unhindered and reaction is fast. |
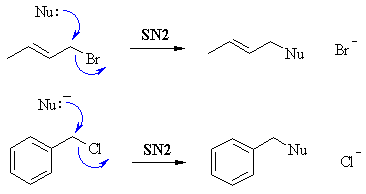 |
| In SN1, the rate determining step is the loss of the leaving group creating the carbocation. The key factor is the stability of the carbocation. Although both are primary (which is normally unfavourable), here both are resonance stabilised. | 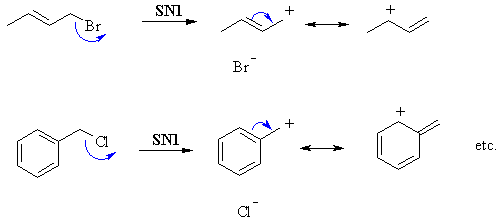 |
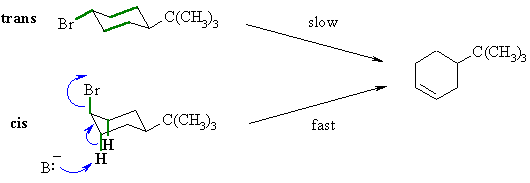
| In the first case, the trans isomer, the bonds that are
antiperiplanar
to the C-Br bond are the two C-C bonds shown in green. Since the ring
flip
is prevented the reaction occurs much more slowly, probably via an E1
reaction.
In the second case, the cis isomer, there are two C-H bonds that are antiperiplanar to the C-Br bond (shown in green) so the E2 is facilitated and occurs rapidly. |
 |