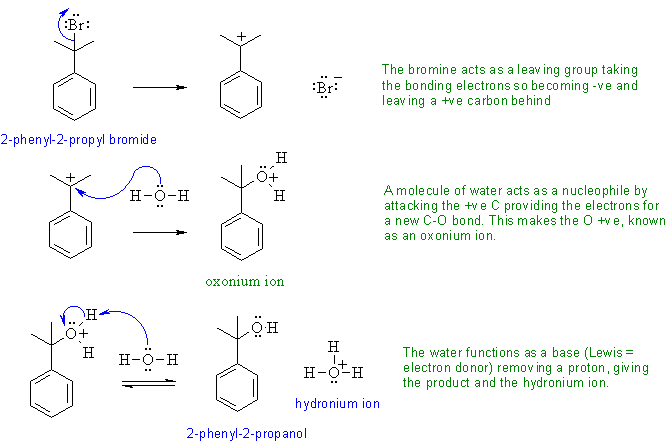
The following diagram shows the solution to the mechanistic question. Note that all the information applies to a single reaction sequence that has been completely described verbally. There is no need for extra reagents or extra steps etc. The curly arrows are drawn specifically to match the text in the question. The biggest problem students have is making sure they understand the language of chemistry. Most students have trouble because they can't draw the structures from the IUPAC names. Read the words carefully, and then make the curly arrows tell that same story. Remember curly arrows go from electron rich to poor and to balance the charges at each step,

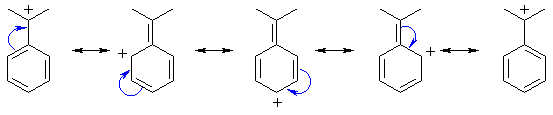
What about the resonance structures of the carbocation ?
A carbocation (read carbo-cation) is just a positively charged carbon atom. Here the cation is benzylic (i.e. adjacent to a benzene ring) so the positive charge can be redistributed around the ring. The curly arrows here should help you derive the structures. All of these are important, only one C in each case lacks an octet and there is no charge separation.