EA:
The data adds up to 100% so there are no "hidden elements" but we don't
know the molecular weight. That doesn't matter because the data can
still
provide the empirical formula (simplest ratio) by considering
using
a 100g and remembering NEVER TO ROUND DATA during EA calculations (it
will
invariably mean you get the wrong answer).
%C = 83.24 divide by atomic weight
: 83.24/12.011 = 6.9303
moles
%H = 16.76 divide by atomic weight : 16.76/1.008 = 16.627
moles
So we have 6.9303 moles of C for every 16.627 moles of H, by dividing by the smallest, that tells us for each C there are 2.4 H atoms.... or C1H2.4 but that is not an empirical formula, the simplest integer ratio is C5H12. The easiest way to get this is to multiply by 10 first to get rid of the decimal then recognise that you can divide by 2.
So the empirical formula = C5H12. Since there are only 3 isomers, this means this is also the molecular formula.

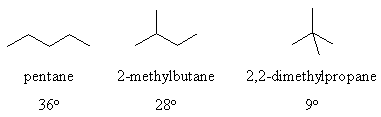
Why the boiling point order ?
Physical properties are determined by intermolecular forces (i.e.
the
forces between molecules) and are NOT connected to the thermodynamic
stability
(which is primarily governed by the intramolecular forces such as the
covalent
bonds).
There are 3 types of intermolecular forces : London dispersion forces,
dipole-dipole forces and hydrogen bonding.
In the alkanes for this question we are only talking about the London
dispersion forces since we lack polar bonds (so no dipole-dipole
interactions)
and no H atoms on electronegative N or O atoms (so no H-bonding).
Non-branched
structures are essentially linear in shape. A more branched structure
is
more spherical in shape and therefore has less surface contacts
with neighbouring molecules. This means there are less
intermolecular
forces and so less energy (hence lower temperature) is
required
to separate the molecules during the boiling process.