Part 8: THERMODYNAMICS
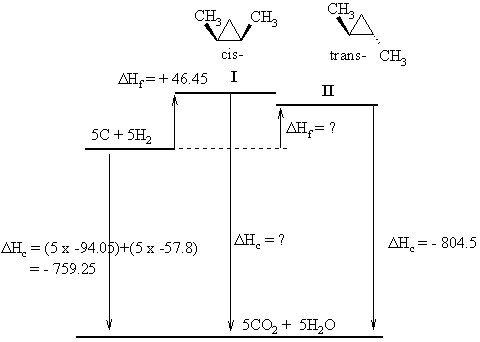 |
The diagram to the left shows the structures of the two
isomers (cis-
means the two methyl groups are on the same face of the ring, trans-
means they are on the opposite face), and it shows the energy
relationships
between the elements, the isomers and their combustion products.
First task is to determine the molecular formula = C5H10
and
use this to calculate the heat of combustion of the elements = - 759.25
kcal/mol.
|
The Hess's Law relationship that comes
from the diagram is : ΔHc
(elements) = ΔHf (isomer)
+
ΔHc (isomer)
So, based on the information for isomer I we
get : - 759.25 = + 46.45 + ΔHc (isomer
I)
Therefore, ΔHc
(isomer
I) = - 759.25 - 46.45 = -805.7 kcal/mol
And for isomer II
we get : - 759.25 = ΔHf
(isomer II) - 804.5
Therefore, ΔHf
(isomer
I) = - 759.25 + 804.5 = + 45.45 kcal/mol
Isomer I is
the cis- isomer and
isomer II is the trans- isomer (which is the more
stable
isomer, see diagram, where it is at a lower energy).
First note that
both systems are eclipsed conformations.
In the cis- isomer the two methyl groups are in a syn
conformation
and are eclipsed with each other leading to torsional strain. In the trans-
isomer the methyl groups are eclipsed with H atoms about 120 degrees
from
each other. This is still destabilising, but because the H atoms are
smaller
than the methyl groups, the steric interaction is less in this case.
Hence
the proximity of the methyl groups in the cis- isomer
destabilises
it compared to the trans- isomer.
(Note : that the
endothermic heats of formation
are real values, it just means the compound is less stable than its
elements,
mainly due to the ring strain)
