Part 7: MECHANISMS
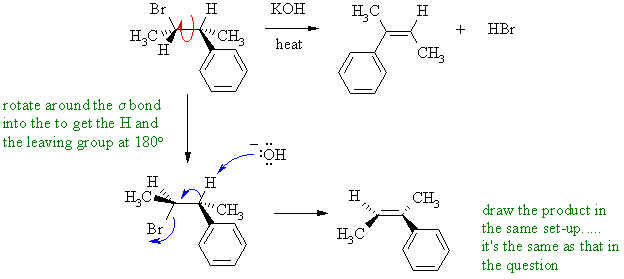
I. Alkyl halide heated with
a strong base to give an alkene
? This is a 1,2-elimination, an example of an E2 reaction. The
important
aspect of an E2 is the anti arrangement (180 degrees) required for the
C-H and the C-LG bonds. In the system shown, rotate the
conformation
to set up the required staggerd conformation with the anti arrangement
and push the arrows give the alkene with the two methyl groups trans-
in the alkene. If you eliminate in the drawn conformation (C-H
and
C-LG at 0 degrees, then the two methyl groups would end up cis-
in the alkene).
II
Change in skeleton suggests carbocations with 1,2-alkyl
shifts and the fact that there are two products with the -OH groups in
different locations suggests 1,2-hydride shifts. No alkene, so
not
an elimination (it's not conc. H2SO4) therefore really
an SN1 reaction (aq. H2SO4). Protonate to make
a better leaving group, generate the C+, rearrange, attack the C+ with
the nucleophile (water not hydroxide since we are under acidic
conditions).
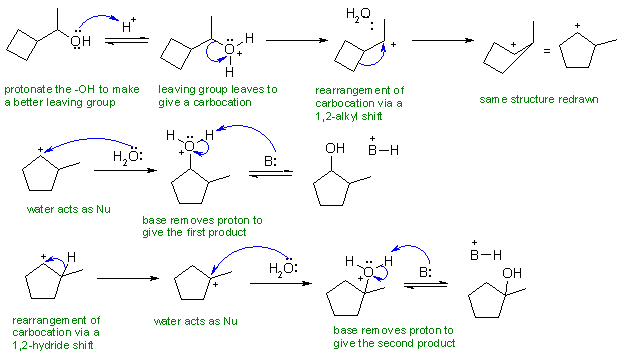
In the scheme B: is used to represent
the bases present... these could be HSO4-, water
or any of the alcohols shown.
III
Alcohols react with alkyl halides to give ethers (Williamson
ether synthesis). So the alcohols cyclohexanol 1
and phenol
2
are the nucleophiles here,
reacting in an SN2 reaction with methyl
iodide to give methoxycyclohexane and methoxybenzene. Resonance
of
the O lone pairs in phenol delocalises the electron density there
making
the phenol less reactive. This is perhaps best
appreciated
by considering the anions. Resonance in the phenoxide ion (left)
delocalises the lone pair making it a weaker nucleophile. There is no
resonance (no pi system) in the alkoxide (right).

IV
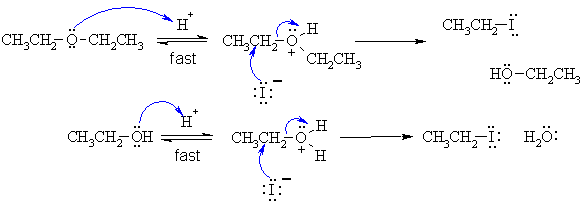
Alcohols react with HI to give alkyl iodides. Remember
that HO- is a poor leaving group. But here we have an ether, and that
means that the potential leaving group is RO- : it's a poor leaving
group too.
Therefore
we must protonate the O atom first to make a better leaving group (in
this
case ROH). Since we have primary systems there will be no
C+
formation (i.e. not SN1) and we get the SN2 reaction with
the iodide
nucleophile displacing the leaving group. The ether cleaves
initially
to give the alkyl iodide and the alcohol. The alcohol then reacts again
in a
similar
fashion to give the second molecule of the alkyl iodide due to the
excess HI.