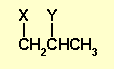Part 8: SPECTROSCOPY
The following data is available from the
question:
MS: M = 198 g/mol (even, m and
m+2, 1:1 ratio is the isotope
pattern for 1 x Br)
EA:
Elemental analysis data for C,H and N (i.e. standard analysis) showed
54.3%
C and 5.57% H
With MW from MS gives the partial molecular formula = C9H11?
(MW = 119)
The E.A. was run for N but none was reported, but the MS shows
Br therefore MF = C9H11Br (MW = 119 + 79
=198)
From here we get the IHD
= 4
IR:
Not
much, C-H above and below 3000cm-1. There are significant
absorptions
at 1600cm-1
due to C=C. There is no OH (about 3500cm-1),
no C=O around 1700cm-1.
13C
nmr: The proton decoupled spectrum shows a total of 7 peaks
indicating
7 types of C. By analysis of the chemical shifts, we have 4 types in
the
region for Ar C 120-140 ppm, 50 and 47 ppm (slightly deshielded) and
those
at 26 ppm are most likely from a hydrocarbon portion.
1H
nmr: First of all we have 4 types of H showing up. After this,
it's a good idea to tabulate the information to make sure you get it
all
correctly matched up:
|
d/ppm
|
multiplicity
|
integration
|
Inference
|
|
7.2
|
"multiplet"
|
5
|
5 Ar-H = monosubstituted benzene |
|
4.3
|
sextet
|
1
|
1 deshielded H with 5H neighbours |
|
3.1
|
doublet
|
2
|
CH2 coupled to 1H, deshielded |
|
1.7
|
doublet
|
3
|
CH3 coupled to 1H |
The most significant structural information.....monosubstituted
aromatic = C6H5-, a CH3CH unit and a
CH next to 5H
Summary....
The MS indicated MW = 198 g/mol and the presence of one Br atom.
The IR showed the presence of C=C (Ar C=C)
13C peaks at 120-140ppm suggests that there is a benzene ring, which is confirmed
by the H NMR, and it's monosubstituted.
H nmr also gives a CH3CH unit with the CH being next to 5H hence
CH3CHCH2
Use this to check the molecular formula : C9H11Br = 9
x 12 + 11 x 1 + 1 x 79 = 198 g/mol
So with all this information we have the following pieces:
C6H5-, CH3CHCH2
and Br
Altogether...
With the pieces we have : C6H5-, CH3CHCH2
and Br
So we have a propane chain with two substituents, the phenyl and the
Br.
All we have to do is decide which is where....
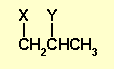
Using the H nmr chemical shift of
the CH group at 4.3ppm, we can conclude that it is the Br that is
attached to the CH (more deshielded due to the electronegative Br).
|
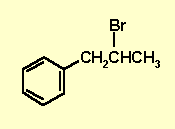
2-bromo-1-phenylpropane
|
The final step should always be to check what you have drawn. The easiest thing
to check is usually the coupling patterns you would expect to see, and the
chemical shifts of each unit.
You should be asking yourself : "Does my answer give me what the H-nmr
shows ?"
Note In the original examination, the CH2 signal
was shown as a triplet (in reality it's even more complex than that if you look
at it at higher frequencies). The unexpected issue here with the triplet is
because the adjacent CH is a chirality center and the coupling of the CH2
to the CH is not simple, because each H in the CH2 is different (diastereotopic)
and each couple to the CH to give a doublet, so it's not a triplet we see, but
really (more like) 2 doublets.