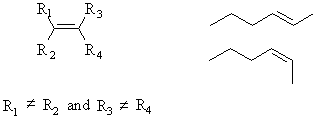
EA:
The data adds up to 100% so there are no "hidden elements" but we don't
know the molecular weight. That doesn't matter because the data can
still
provide the empirical formula (simplest ratio) by considering
using
a 100g and remembering NEVER TO ROUND DATA during EA calculations (it
will
invariably mean you get the wrong answer).
%C = 85.63 divide by atomic weight
: 85.63/12.011 = 7.129
moles
%H = 14.37 divide by atomic weight : 14.37/1.008 = 14.256
moles
So we have 7.129 moles of C for every
14.256 moles of H, by dividing
by the smallest, that tells us for each C there are 2.0 H atoms.... or
C1H2
Hence empirical formula, the simplest integer ratio is CH2.
So if we have 6 C atoms, then the
molecular formula = C6H12.
Remember isomerism is a pair relationship and the compounds must
have the same molecular formulae.
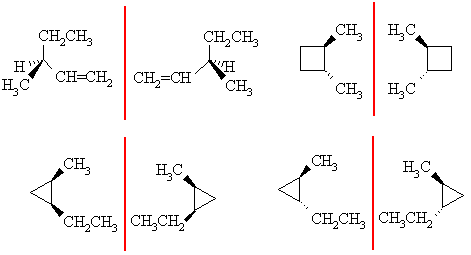
Enantiomers are non-superimposable mirror images, for that they need a chirality center, which is this case needs to be an sp3 C with 4 different substituents attached. There are three possible pairs for C6H12:

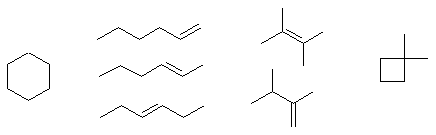
Constitutional isomers contain different "pieces" having either different functional groups (e.g. alkene and cycloalkane here) or different connectivity orders. There are lots of possibilities here, a small selection of compounds are shown below, any pair from this list would be constitutional isomers:
Geometric isomers are cis- or trans- alkenes. In order for this to be possible, the two substiutents at each end of the double bond must be different.