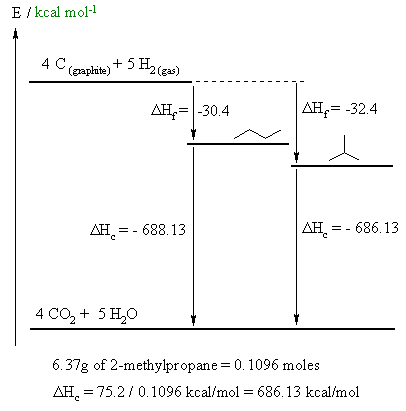
A fairly straight forward calculation, but care needs to be taken to draw the diagram correctly and making sure you do the math correctly.
A. 4 C (graphite) + 5 H2 (gas) --> CH3CH2CH2CH3 (gas)
B. (CH3)3CH (gas) + 13/2 O2 (gas) --> 4 CO2 (gas) + 5 H2O (gas)
C.

D. The calculation is shown on the figure above. The steps are :
(i) calculate the number of moles of 2-methylpropane in 6.37g
(ii) calculate the ΔHc / mol for 2-methylpropane by dividing the heat given by part (i)
(iii) calculate the ΔHc / mol for butane by recognising that ΔHf + ΔHc must be the same for the two isomers.