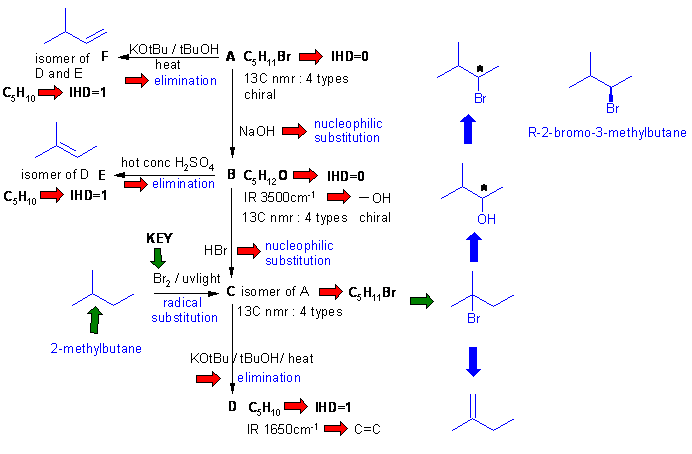
Important points:
(1) C is obtained from 2-methylbutane with Br2 / uv tells you that C is 2-bromo-2-methylbutane (3o H most reactive).
(2) Formation of C from B with HBr results in a rearrangement because reaction with PBr3 gives A.
(3) t-butoxide eliminations (tBuO-) often disobey Zaitsev's rule - remove least hindered H, hence C to D.
(4) Alcohol dehydration obeys Zaitsev's rule - hence B to E (more substituted = more stable alkene).
(5) As D, E and F react to give the same alkane, F is deduced (only other alkene variant).
(6) A can be deduced from knowing B or F or based on the chirality information.
(1) C is obtained from 2-methylbutane with Br2 / uv tells you that C is 2-bromo-2-methylbutane (3o H most reactive).
(2) Formation of C from B with HBr results in a rearrangement because reaction with PBr3 gives A.
(3) t-butoxide eliminations (tBuO-) often disobey Zaitsev's rule - remove least hindered H, hence C to D.
(4) Alcohol dehydration obeys Zaitsev's rule - hence B to E (more substituted = more stable alkene).
(5) As D, E and F react to give the same alkane, F is deduced (only other alkene variant).
(6) A can be deduced from knowing B or F or based on the chirality information.