| With the pieces we have : H-C=C-H, CH3CH2CH2
and CO2H We actually have the structure as we only have 1 middle piece, the H-C=C-H and two then end pieces. |
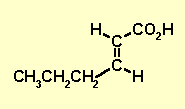 2-hexenoic acid |
The following data is available from the
question.
Note
: Remember to cross
reference things to confirm.... eg. IR mnay show C=C, use NMR to
confrim that.
MS: M+ seen at 114 g/mol (even MW, no Cl or Br isotope patterns).
EA:
Elemental analysis data for C and H (i.e. standard analysis) showed
63.14%
C and 8.83% H (therefore there is 28.03% something else, most
likely oxygen : remember oxygen can't be detected in a combustion
analysis). Use the MW from the mass spectra (above) to get
the partial molecular formula = C6H10 ?
(adds up to 72 + 10 = 82 so that leaves 32). The missing piece is
consistent with 2 oxygen atoms (because 2 x 16 = 32) .... hence
C6H10O2
From here we get the IHD
= 2
IR: Confirms the presence of oxygen. There is a C=O at about 1700 cm-1 and a typical broad OH band between 3400-2300cm-1. C-O can be seen around 1300cm-1. There is also a band to the right of the C=O at about 1650cm-1 to consider, a possible C=C.
13C nmr: The proton decoupled spectrum shows a total of 6 peaks indicating 6 types of C. By analysis of the chemical shifts, we have types in the region for 1 x C=O above 160 ppm, two types of C=C at 100-160ppm, and 3 peaks between 0-40 ppm that are most likely from a hydrocarbon portion.
1H nmr: First of all we have 6 sets of peaks so we have 6 types of H showing up. After this, it's a good idea to tabulate the information to make sure you get it all correctly matched up:
|
|
|
|
|
|
|
|
|
exchangeable => OH probable carboxylic acid RCO2H |
| 7.2 |
|
|
alkene C=C complex coupling (doublet of triplets) to 2H and 1H |
| 5.8 |
doublet |
1 |
alkene C=C coupled to 1H |
| 2.2 |
quartet |
2 |
CH2 coupled to 3H, slightly deshielded |
|
|
|
|
CH2 coupled to 5H |
|
|
|
|
CH3 coupled to 2H |
| With the pieces we have : H-C=C-H, CH3CH2CH2
and CO2H We actually have the structure as we only have 1 middle piece, the H-C=C-H and two then end pieces. |
 2-hexenoic acid |
The final step should always be to check
what you have drawn. The
easiest
thing to check is usually the coupling patterns you would expect to
see,
and the chemical shifts of each unit.
You should be asking yourself : "Does my answer give me what the
H-nmr shows ?"
Notes
: