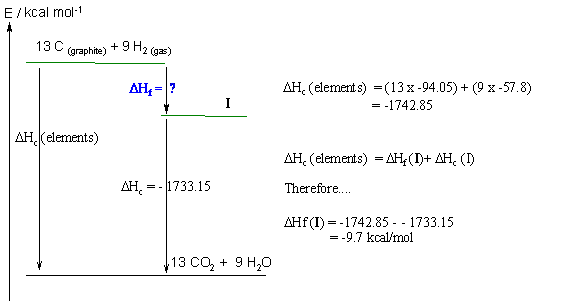
Part 8: THERMODYNAMICS
This should have been a
reasonably straight forward
calculation, but care needs to be
taken
to get the right structure and the right molecular formula, then do the
math correctly.
Common stumbling blocks for
some
students were :
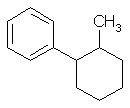
- could not draw 1-methyl-2-phenylcyclohexane and deduce the
correct molecular formula, C13H18
- failed to draw clear diagrams of the chair conformations with the
axial and equatorial positions clearly shown.
- failed to draw 1,2- configurational isomers because they did not
know their isomer types.
If we are talking about configurational
isomers of 1-methyl-2-phenylcyclohexane, we must
be talking about the cis-
and trans- systems. We are
not talking about chair
and boat isomers (these would
be conformational
isomers) or other substitution patterns including different branching
patterns
(these would be constitutional
isomers).
(note : in order to
clearly show the required isomers the chair structures with the axial
and equatorial substituents needed to be shown. Some students drew
diagrams that were indistinguishable from each other e.g. planar
cyclohexanes with wedges and hashes to denote up and down. This was
insufficient for full marks.)
The
question asks for an analysis of
the
conformations.
Axial substituents are destablised by Van
der Waals interaction and some torsional strain
with the adjacent C-C. The Van
der Waals interaction between
the axial position and the other
axial
substituents on that face (in this case these are both H atoms). These
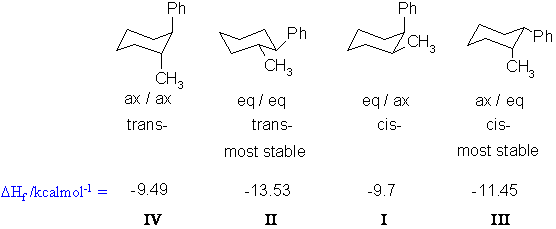
are examples of 1,3-diaxial interactions. Hence
substituents generally prefer to be equatorial where there is no such
destabilisation. The most stable
system here
will
be the trans- isomer where boths groups are
equatorial. The most stable cis isomer will be the one with the
larger phenyl group in the preferred equatorial postion and the smaller
methyl group axial. In all cases the chair conformations are
preferred since they are totally staggered and are strain free.
(note : many students
made sweeping statements about trans being more stable than cis others
said the left most structure was the most stable. Neither are true. )