Part 8: THERMODYNAMICS
This should have been a
reasonably straight forward
calculation, but care needs to be
taken
to get the right structures and the right molecular formula, then do
the
math correctly.
Common stumbling blocks for
some
students were :
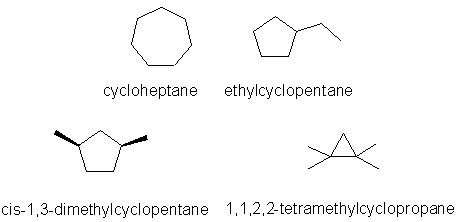
- could not draw cis-1,3-dimethylcyclopropane correctly.
- could not deduce the
correct molecular formula, C7H14
- failed to assign stability order correctly because they did not
consider ring strain versus branching issues.
a.
ii
All are isomers of C7H14.
iii Constitutional
isomers
b.
c.
i Remember
that the more stable the isomer the more exothermic the heat of formation will
be.
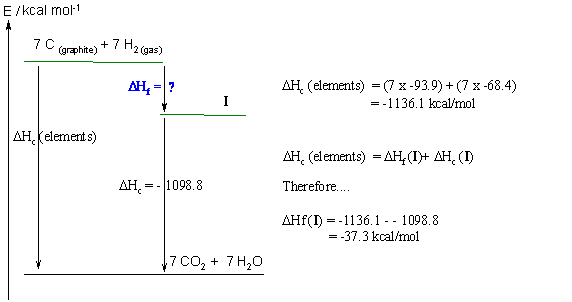
Hence the least stable isomer, IV (1,1,2,2-tetramethylcyclopropane) = -28.6
kcal/mol
The most stable isomer, III (cis-1,3-dimethylcyclopentane) = -40.2
kcal/mol
and finally, the intermediate isomer, II (ethylcyclopentane) = -39.1 kcal/mol
ii cis-1,3-dimethylcyclopentane
iii 1,1,2,2-tetramethylcyclopropane
d.
The
question asks for the main factors affecting the stability of alkanes
and cycloalkanes. The factors are branching and ring strain (which is
due mainly to angle and torsional strains), plus other torsional
strains and maybe Van der Waals strain. Remember that steric
strain is the overall effect of angle, torsional and Van der Waals
strain. Note that heats of formation or combustion etc. do not affect stability.
They are measures
of stability. The stability controls the heat of reaction not the other
way around.
Since the ring strain of cyclopentanes and cycloheptanes are about the
same the difference in the stability of these structures is controlled
by the degree of branching. The methyl groups in
cis-1,3-dimethylcyclopentane are too
far apart to suffer Van der Waals
strain or torsional strain (look at a model of the most stable
conformation - the two methyl groups are both in the equatorial type
positions). Therefore, this more branched isomer
dimethyl isomers is more stable than the ethyl isomer.
In contrast, the
1,1,2,2-tetramethylcyclopropane has a lot of ring
strain due to angle strain (bond angle of 60 degrees compared to ideal
109.5) and torsional strain within the ring and between the
methyl substituents, hence it is the least stable. Ring strain is
small rings like cyclopropane and cyclobutane is very large compared
the to the stabilisation given by simple branching.