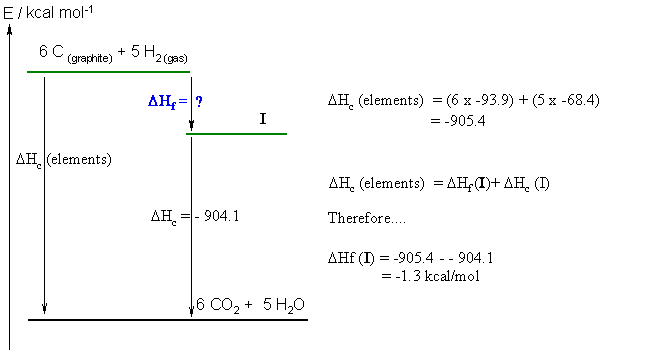
c. First step... the molecular formula = C6H10

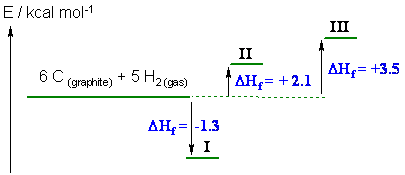
d.
i (just calculated in c) = -1.3 kcal/mol, ii = + 2.1 kcal/mol and
iii = +3.5 kcal/mol
Hence i is the most stable
(it has the most exothermic heat of formation) and iii is the least stable
(you were not expected to draw this diagram but it helps show which is the most
and which is the least stable).
e. The 3D models shown in the question show that cyclopentene is a planar structure. This means that the CH2 units are eclipsed. If we look at the right figure in the question we can see that the vinyl H (i.e. the H on the C=C unit) not eclipsong the adjacent H. Therefore if the vinyl H is replaced with a methyl group as in i, then the methyl group is staggered with respect to the H. In ii the methyl group will be eclipsing one H atom on the adjacent sp3C atom while in iii the methyl group will be eclipsing two adjacent H atoms, one of each of the two adjacent sp3C atoms so iii has the most eclipsing interactions and is therefore the least stable.
Common stumbling blocks for some students were :