Part 9 : STRUCTURE DETERMINATION
Note that these types of questions are often the most difficult on the examination.
This is because they are holistic in that they require a good understanding
of the reactions and the concepts and the ability to apply those concepts. They
can't be memorised, they require understanding and ability to apply. Hence they
help discriminate those who truly understand from those who have memorised and
will soon forget.
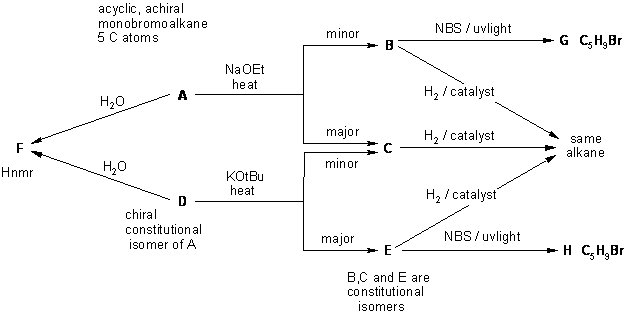
A full schematic of the full solution is presented below. This is also presented
in several parts, where each diagram has more information added to it.
The idea behind this is to encourage students to see how to work towards the
answer by building up the answer from the information in the question gradually.

Diagram 2 gives the basic information from the question in black and deductions directly from this information are indicated by red arrows and with blue text plus it identifies the functional groups and points that provide potential key information by green arrows and key initial structures. Here, the interpretation of the HNMR data to deduce F is key information. The HNMR shows 2 methyl groups that are singlets, the coupling for an ethyl group and the exchangeable H of an alcohol group.
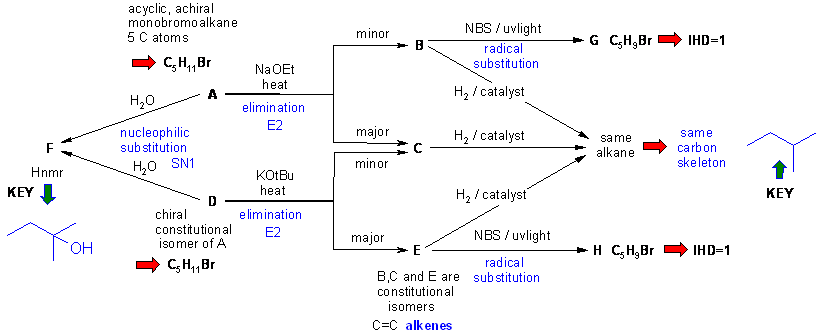
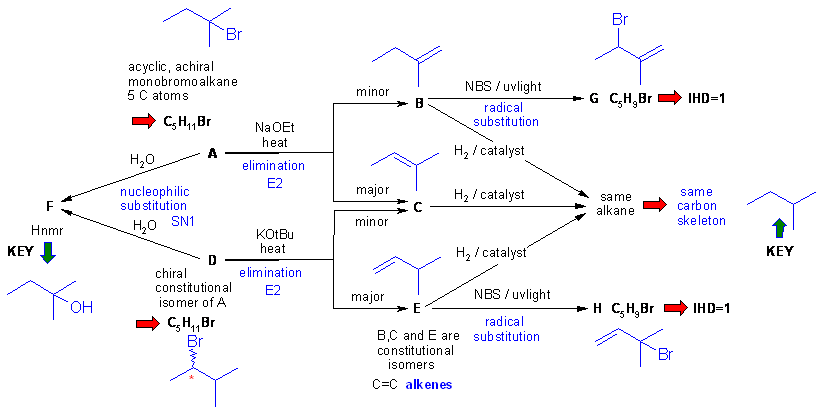
Diagram 3
gives the completed solution with the structures in blue. In this problem
the are several routes to connect the structures.
Key steps are recognising F then using the skeleton of F as the framework for B, C and E, the chirality information then allows A and D to be deduced, then B, C and E based on Zaitsev's rule etc. Finally G and H are deduced as the products to allylic radical substitutions.
Common errrors:
Could not determine chiral centers, poor interpretation of H NMR, did not know what D2O exchange means, did not know the meaning of acyclic or achiral, did not use wedge/hash diagrams to show 3D structures, did not know what geometric or configurational isomers are, could not determine R/S or E/Z configurations.