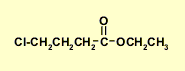Part 8: SPECTROSCOPY
The following data is available from the
question.
Note
: Remember to cross
reference things to confirm.... e.g.
IR may show C=C, use NMR to
confirm that etc.
MS: M+
seen at 150 g/mol but also an m+2 at 152. The 3:1 ratio of m : m+2 implies a
Cl isotope patterns. MW is even, so N rule implies no N (or an even number of
N atoms)
IR:
There is a C=O at 1740 cm-1 which is a little high for a simple
ketone. The only other absorbances of any note are 1214 and 1149 cm-1
which are probably C-O.
13C nmr:
The proton decoupled spectrum shows a total of 6 peaks indicating 6 types of
C. By analysis of the chemical shifts, we have a C=O at 173ppm, suggesting a
carboxylic acid derivative, peaks at about 60ppm and 44ppm (possibly carbons with an
electronegative atoms attached) and then 3 peaks between 0-40 ppm that are
most likely from a hydrocarbon portion.
1H nmr:
The proton spectrum shows a total of 5 peaks indicating 5 types of H.
|
d/ppm
|
multiplicity
|
integration
|
Inference
|
|
4.2
|
q
|
2
|
CH2 coupled to 3H, deshielded
|
|
3.6
|
t
|
2
|
CH2 coupled to 2H, deshielded
|
|
2.5
|
t
|
2
|
CH2 coupled to 2H, deshielded
|
|
2.2
|
p
|
2
|
CH2 coupled to 4H |
|
1.3
|
t
|
3
|
CH3 coupled to 2H
|
(p
= pentet, q = quartet, t = triplet, d = doublet, s = singlet)
The most significant structural information
from this are:
- two hydrocarbon units: CH3CH2- and -CH2CH2CH2-
Summary....
The MS indicated MW = 150 and Cl
The IR showed the presence of C=O, possible
C-O
13C NMR confirms C=O and suggests a carboxylic acid derivative. A C-O is also possible
H NMR gives CH3CH2- and -CH2CH2CH2-
This
is in agreement with a molecular formula of C6H11O2Cl
with MW = (yes!)
Altogether...
|
The fragments we have are:CH3CH2- and -CH2CH2CH2-,
C=O, -O- and -Cl
The chemical shift of the CH2
in the CH3CH2 group requires that it be attached
to the O, (it can't be the -Cl), so we have an ethoxy group, CH3CH2O-
The IR and C-NMR data suggest that an ester is present.
This implies that one -CH2- in the -CH2CH2CH2- group is connected to the C=O and the other to the Cl.
|
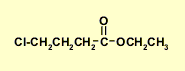
ethyl 4-chlorobutanoate
|
The final step should always be to check what you
have drawn. The easiest thing to check is usually the coupling patterns you
would expect to see, and the chemical shifts of each unit. You should
be asking yourself : "Does my answer give me what the H-nmr shows ?"