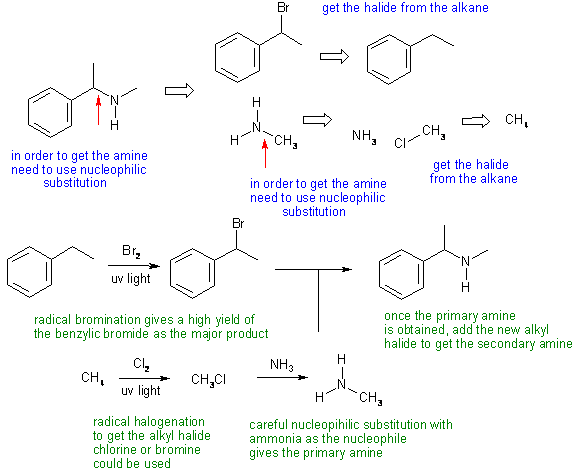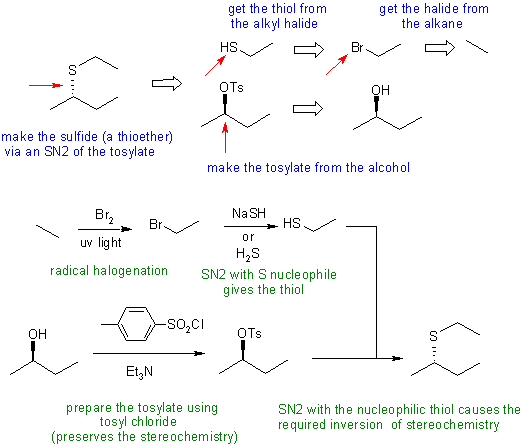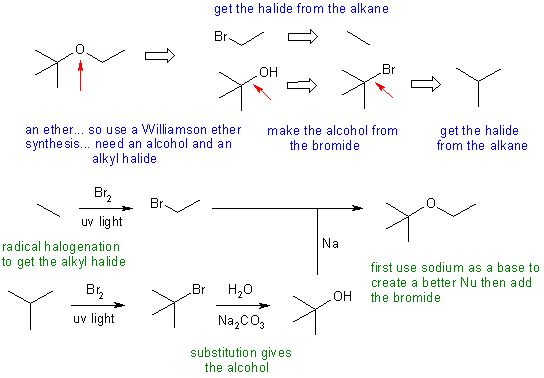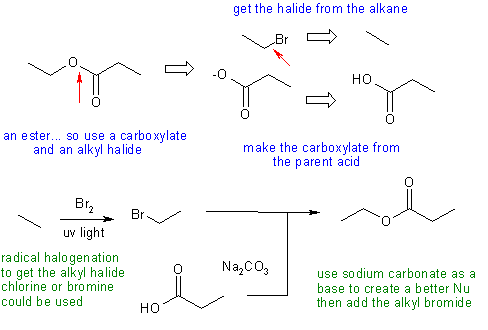
Answers to the synthesis problems are given below. These answers have been selected as they are short but there are probably other reasonable / alternative solutions ..... Of course they are based on the reactions covered in the Fall semester !
Common errrors:
Not reading the question and hence not showing how to make any materials that contribute C to the product from the allowed starting materials.... the one specified in each part plus any hydrocarbon with three carbons or less.Trying to react alkanes with Nu like ammonia or water without first radically halogenating to introduce a leaving group. Confusing reagents.
A: Not using a suitable base to deprotonate the acid to make a more nucleophilic carboxylate (good choices = Na2CO3 or HO-, poor choices = Na or NaNH2 (too strong = dangerous!)). Trying to react a carboxylate with an alcohol (both are Nu).
B: Using the wrong amine or halide in the required nucleophilic substitution reactions.
C: Ignoring the stereochemistry or getting the stereochemistry wrong by attempting a double inversion when only one is needed. We also need to avoid SN1 conditions (e.g. acids such as H2SO4 or HBr) and the associated carbocation to avoid any loss of stereochemistry - this means not using an acidic route to converting the -OH to a better leaving group. Poor reagent selection when preparing the thiol.
D: Poor tracking of the number of C atoms in structures... i.e. missing and / or gaining C carelessly.