Part 6: SPECTROSCOPY
Note
: Remember to cross
reference things to confirm.... e.g.
IR may show C=C, use NMR to
confirm that etc.
The following data is available from the
question.
MF was provided : C11H15NO
MS: Since the MF was provided, the MS is probably of limited (if any) value.
IR:
There is a C=O at just below 1700 cm-1 which is a little low for a simple
ketone. There is no sign of either -OH or -NH (above 3200 cm-1).
13C nmr:
The proton decoupled spectrum shows a total of 7 peaks indicating 7 types of
C. By analysis of the chemical shifts, we have a C=O at 190ppm (this is borderline and so it's not easy to say what subtype of C=O at this stage), 4 peaks between 110-155 ppm that are 4 aromatic C, 1 peak at about 47ppm (possibly a C with an
electronegative atom attached) and then 1 peak at 13 ppm that is
most likely from a hydrocarbon portion.
1H nmr:
The proton spectrum shows a total of 5 peaks indicating 5 types of H.
|
d/ppm
|
multiplicity
|
integration
|
Inference
|
| 9.7 |
s |
1 |
aldehyde H |
|
7.7
|
"d"
|
2
|
2 ArH
|
|
6.7
|
"d"
|
2
|
2 ArH
|
|
3.5
|
q
|
4
|
2 x CH2 coupled to 3H, deshielded
|
|
1.3
|
t
|
6
|
2 x CH3 coupled to 2H
|
(m
= multiplet, q = quartet, t = triplet, d = doublet, s = singlet)
The most significant structural information
from this are:
- two hydrocarbon units: a disubstituted benzene ring, C6H4 and 2 equivalent CH3CH2 groups.
- the presence of the aldehyde group, HC=O
Summary....
The molecular formula was provided :C11H15NO
Based on the molecular formula, IHD = 5
The IR showed the presence of C=O, H NMR tells us that it is an aldehyde RCHO (9-10ppm).
13C and H NMR gives a disubstituted aromatic, C6H4, and tells us that it is a para substitution pattern (number of Ar C and Ar H types).
The C=O and the benzene ring account for the IHD of 5.
Altogether...
|
The fragments we have are para disubs benzene -C6H4 -, HC=O, N and 2 x - CH2CH3
The only one way that these pieces can be assembled .... a substituted aminobenzaldehyde
The H NMR for the ethyl groups is consistent with them being attached to a N atom. |
p-N,N-diethylaminobenzaldehyde
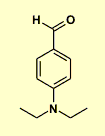
|
The final step should always be to check what you
have drawn. The easiest thing to check is usually the coupling patterns you
would expect to see, and the chemical shifts of each unit. You should
be asking yourself : "Does my answer give me what the H-nmr shows ?
