Part 7: MECHANISMS
Note that no other reagents are needed in order to complete any
of these sequences, you should only be using what is there.
Common errrors:
A: Showing the reactions as SN2 or E2 instead of carbocation based SN1 or E1. Not counting C atoms correctly, not protonating the OH to make a better leaving group and not identifying the base.
B: Not interpretting the Newman projection correctly, ignoring the stereochemistry of the E2 pathway, showing an E1 pathway via a carbocation.
C: Making the sp2 aromatic C undergo SN1 or SN2 reaction. Showing an SN1 reaction of a primary carbocation.
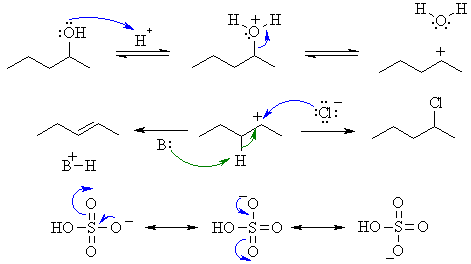
A
Here we have two acids reacting with an alcohol but one giving elimination to the alkene, the other giving substitution to an alkyl chloride. The difference arises because of a difference in the conjugate bases of the acids. Both reactions occur via protonation of the OH to make a better leaving group followed by loss of the leving group to give an intermediate carbocation. Then in the SN1 process, the chloride ion functions as a nucleophile, and attacks the carbocation intermediate giving substitution but the hydrogen sulphate ion is resonance stabilised (charge delocalised) and is a poor nucleophile, and a base removes a proton (E1) generating the alkene. The base could be the alcohol, hydrogen sulphate or a water molecule.

B
If an alkyl bromide is heated with strong base (here it's ethoxide), then a 1,2-elimination occurs to give an alkene. Alkyl halide eliminations are typically E2. This prefers an anti arrangement of the C-H and C-LG bonds. In this reaction, the base is hydroxide (i.e. small), so the Zaistev elimination is going to dominate to give the more highly substituted alkene and the major product will be the more stable trans-alkene from the more stable conformation (methyl groups anti).
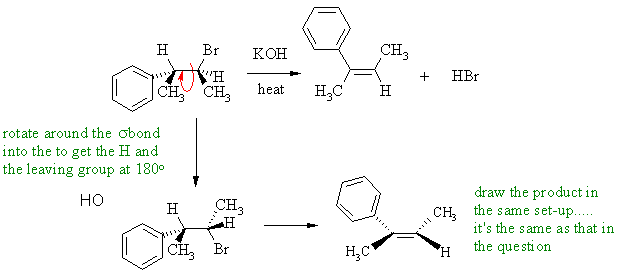
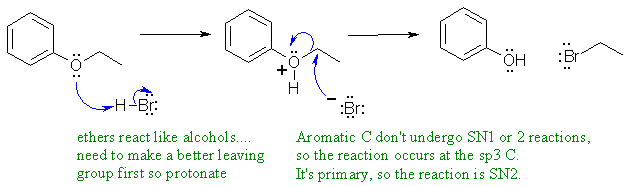
C
Ethers react with strong acids like alcohols... need to make a better leaving group by protonating.